Antioxidant and antibacterial activity of extract and phases from stems of Spartium junceum L. growing in Algeria
Keywords:
Spartium junceum L., Phenolic compounds, Antioxidant activity, Antibacterial activityAbstract
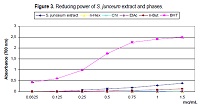
This work aimed to evaluate the antioxidant and antibacterial activities of the hydroalcoholic (80% methanol) extract and n-hexane (n-Hex), chloroform (Chl), ethyl acetate (EtAc), and n-butanol (n-But) phases from Spartium junceum L. stems collected inAlgeria. Preliminary phytochemical investigations on phenolic compounds have been carried out. The total phenolic content, spectrophotometrically determined, ranged from 71.8095 ± 3.7136 mg GAE/g (extract) to 0.0582 ± 0.0106 mg GAE/g (n-Hex). By HPLC-PDA analysis flavonoids (flavone derivatives), p-hydroxybenzoic acid, p-hydroxycinnamic acid, and cinnamic acid derivatives were identified both in the extract and phases. S. junceum extract showed a noticeable free radical scavenging effect in the DPPH test (IC50 = 0.6833 ± 0.0240 mg/mL), mild reducing power, and strong chelating activity (IC50 = 0.2292 ± 0.0138 mg/mL). Among the phases, n-But displayed the best effect both in the DPPH test and reducing power assay, whereas n-Hex resulted the most active in the ferrous ions chelating activity assay. A positive relationship between DPPH radical scavenging activity and total phenolic content was found. Both the extract and phases exhibited antimicrobial activity against Gram-positive bacteria only. Staphylococcus aureus ATCC 6538 was the most susceptible strain (MIC range: 15.60-250.00 µg/mL), and the Chl phase showed the greatest efficacy. S. junceum extract resulted non-toxic against Artemia salina. The obtained results demonstrate the potential of S. junceum stems as safe sources of natural antioxidant and antimicrobial compounds.
References
. Millspaugh CF. American Medicinal Plants. General publishing Co. Toronto;1974.
. Heywood VH. Spartium L.. In: Tutin TG, Heywood VH, Burges NA, Moore DM, Valentine DH, Walters SM, Webb DA, editors. Flora Europea. Vol. II. Cambridge at the University Press; 1968. p. 101.
. Yesilada E, Takaishi Y, Fujita T, Sezik E. Anti-ulcerogenic effects of Spartium junceum flowers on in vivo test models in rats. J Ethnopharmacol. 2000;70(3):219-226.
. Carmona MD, Llorach R, Obon C, Rivera D. Zahra, a Unani multicomponent herbal tea widely consumed in Syria: components of drug mixtures and alleged medicinal properties. J Ethnopharmacol. 2005;102:344-350.
. Bussmann RW, Glenn A. Fighting pain: Traditional Peruvian remedies for the treatment of asthma, rheumatism, arthritis and sore bones. Indian J Tradit Know. 2011;10(3):397-412.
. Guarrera PM, Salerno G, Caneva G. Folk phytotherapeutical plants from Maratea area (Basilicata, Italy). J Ethnopharmacol. 2005;99(3):367-378.
. Scherrer AM, Motti R, Weckerle CS. Traditional plant use in the areas of Monte Vesole and Ascea, Cilento National Park (Campania, Southern Italy). J Ethnopharmacol. 2005;97:129-143.
. Mosaddegh MH, Ghasemi N, Mosaddegh A, Hejazian SH. Antinoniceptive effects of Spartium junceum L. extract on mouse formaline test. World Appl Sci J. 2008;3(2):223-226.
. Cerchiara T, Blaiotta G, Straface VS, Belsito E, Liguori A, Luppi B, Bigucci F, Chidichimo G. Biological activity of Spartium junceum L. (Fabaceae) aromatic water. Nat Resour. 2013;4:229-234.
. Yesilada E, Tsuchiya K, Takaishi Y, Kawazoe K. Isolation and characterization of free radical scavenging flavonoid glycosides from the flowers of Spartium junceum by activity-guided fractionation. J Ethnopharmacol. 2000;73:471-478.
. Gao X, Ohlander M, Jeppsson N, Bjork L, Trajkovski V. Changes in antioxidant effects and their relationship to phytonutrients in fruit of the sea buckthorn (Hippophae rhamnoides L.) during maturation. J Agric Food Chem. 2000;48(5):1485-1490.
. Ohnishi M, Morishita H, Iwahashi H, Shitzuo T, Yoshiaki S, Kimura M, Kido R. Inhibitory effects of chlorogenic acids on linoleic acid peroxydation and haemolysis. Phytochemistry. 1994;36(3):579-583.
. Oyaizu M. Studies on products of the browning reaction. Antioxidative activities of browning reaction products prepared from glucosamine. Jpn J Nutr. 1986;44(6):307-315.
. Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agric Food Chem. 1990;38:674-677.
. Bisignano C, Filocamo A, Ginestra G, Giofrè SV, Navarra M, Romeo R, Mandalari G. 3,4-DHPEA-EA form Olea europea L. is effective against standard and clinical isolates of Staphylococcus sp.. Ann Clin Microbiol Antimicrob. 2014;13(24):1-4.
. Meyer BN, Ferrigni NR, Putnam JE, Jacobson LB, Nichols DE, McLaughlin JL. Brine shrimp: A convenient general bioassay for active plant constituents. Planta Med. 1982;45(5):31-34.
. Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteau reagent. Methods Enzymol. 1999;299:152-178.
. Roginsky V, Lissi EA. Review of methods to determine chainbreaking antioxidant activity in food. Food Chem. 2005;92:235-254.
. Nilsson J, Pillai D, Önning G, Persson C, Nilsson Å, Åkesson B. Comparison of the ABTS and FRAP methods to assess the total antioxidant capacity in extracts of fruit and vegetables. Mol Nutr Food Res. 2005;49:239-246.
. Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends Plant Sci - Reviews. 1997;2(4):152-159.
. Salazar R, Pozos ME, Cordero P, Pérez J, Salinas MC, Waksman N. Determination of the antioxidant activity of plants from Northeast Mexico. Pharm Biol. 2008;46:166-170.
. Taviano MF, Marino A, Trovato A, De Pasquale R, Miceli N. Antioxidant and antimicrobial activities of berries extracts of five Juniperus species under Juniperus Section from Turkey. In: Hai-Xue Kuang editor. Phytochemicals: Occurrence in Nature, Health Effects and Antioxidant Properties. Nova Science Publishers, Inc. New York; 2013. p. 55-68.
. Taviano MF, Marino A, Trovato A, Bellinghieri V, La Barbera TM, Güvenç A, Hürkul MM, De Pasquale R, Miceli N. Antioxidant and antimicrobial activities of branches extracts of five Juniperus species from Turkey. Pharm Biol. 2011;49(1):1014-1022.
. Lue B-M, Skall Nielsen N, Jacobsen C, Hellgren L, Guo Z, Xu X. Antioxidant properties of modified rutin esters by DPPH, reducing power, iron chelation and human low density lipoprotein assays. Food Chem. 2010;123(2):221-230.
. Vladimir-Knežević S, Blažeković B, Štefan MB, Babac M. Plant polyphenols as antioxidants influencing the human health. In: Rao V, editor. Phytochemicals as nutraceuticals - Global approaches to their role in nutrition and health. InTech Publisher; 2012. p. 155-180.
. Halliwel B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch Biochem Biophys. 2008;476(2):107-112.
. Barreca D, Bellocco E, Laganà G, Ginestra G, Bisignano C. Biochemical and antimicrobial activity of phloretin and its glycosilated derivatives present in apple and kumquat. Food Chem. 2014;160:292-297.
. Haug T, Stensvåg K, Olsen ØM, Sandsdalen E, Styrvold OB. Antibacterial activities in various tissues of the horse mussel, Modiolus modiolus. J Invertebr Pathol. 2004;85(2):112-119.