Identification of natural products and their derivatives as promising inhibitors of protein glycation with non-toxic nature against mouse fibroblast 3T3 cells
Keywords:
Protein glycation, diabetic complications, antiglycation agents, medicinal plants, cytotoxicity, terpenoids, alkaloidsAbstract
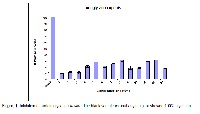
This study was performed to identify new inhibitors of protein glycation in vitro. Protein glycation is one of the major causes of late diabetic complications. In this study, terpenoids and alkaloids, isolated from different medicinal plants, along with their derivatives, were evaluated for their antiglycation activity in vitro, while MTT assay on mouse fibroblast 3T3 cells was used to assess their potential cytotoxicity. Among the tested compounds, gossypol (2,2′-bis-(formyl-1,6,7-trihydroxy-5-isopropyl-3-methylnaphthalene) (1), isolated from Gossypium herbaceum, and its derivatives, gossypol acetic acid (2), gossypolidene- 4-aminoantipyrine (4), and gazolidone (6), showed a potent antiglycation activity (IC50 < 16 µM), while gossypolidene-4-aminoantipyrine (5) showed a significant antiglycation activity with IC50 value 82.934±2.924 µM, in BSA-fluorescence assay. Alkaloid, noscapine (3S)-6,7-Dimethoxy-3-[(5R)-4-methoxy-6-methyl-5,6,7,8-tetrahy-dro-1,3-dioxolo[4,5-g]isoquinolin-5-yl] isobenzofuran-1(3H)-one (7), isolated from Papaver somniferum, N-nitrosoaphyllinic acid (9), a derivative of alkaloid aphylline, and 2H-quinolizine, octahydro salt (11), a salt of alkaloid lupinine, exhibited significant inhibition activity with IC50 values 152.662±5.432, 393.758 ±4.001 µM and 110.203±4.816µM, respectively. Similarly, compounds gossypolidene thiocarbamide (3), deoxypeganine hydrochloride (8), lupinine (10) and cytisine (12) showed moderate inhibition with IC50 values of 401.865 ±18.450, 863.322 ±6.415, 712.176±7.745, and 728.462±2.331 µM, respectively. The results were compared with the standard antiglycation agent, rutin (13) (IC50 =98.012±2.030 µM). Cellular cytotoxicity assay showed only gossypol acetic acid (2) and gossypolidene thiocarbamide (3) as somewhat toxic to 3T3 (mouse fibroblast) cells with IC50 values 2.07 ±0.61 and 5.00 ±1.89 µM, respectively. Cycloheximide was used as a standard in this assay with IC50 value 0.3 ± 0.089 μM
References
. Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytotherapy Research. 2008;22(2):228-37.
. Baynes JW, Watkins NG, Fisher CI, Hull CJ, Patrick JS, Ahmed MU, Dunn JA, Thorpe SR. The Amadori product on protein: structure and reactions. Progress in clinical and biological research. 1989;304:43.
. Bry L, Chen PC, Sacks DB. Effects of hemoglobin variants and chemically modified derivatives on assays for glycohemoglobin. Clinical Chemistry. 2001;47(2):153-63.
. Graves DT, Liu R, Alikhani M, Al-Mashat H, Trackman PC. Diabetes-enhanced inflammation and apoptosis—impact on periodontal pathology. Journal of dental research. 2006;85(1):15-21.
. Reddy VP, Beyaz A. Inhibitors of the Maillard reaction and AGE breakers as therapeutics for multiple diseases. Drug discovery today. 2006;11(13):646-54.
. Singh R, Barden A, Mori T, Beilin L. Advanced glycation end-products: a review. Diabetologia. 2002;44(2):129-46.
. Bendayan M. Immunocytochemical detection of advanced glycated end products in rat renal tissue as a function of age and diabetes. Kidney international. 1998;54(2):438-47.
. Simm A, Wagner J, Gursinsky T, Nass N, Friedrich I, Schinzel R, Czeslik E, Silber RE, Scheubel RJ. Advanced glycation endproducts: a biomarker for age as an outcome predictor after cardiac surgery?. Experimental gerontology. 2007;42(7):668-75.
. Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. New England Journal of Medicine. 1988;318(20):1315-21.
. Wu CH, Huang SM, Lin JA, Yen GC. Inhibition of advanced glycation endproduct formation by foodstuffs. Food & function. 2011;2(5):224-34.
. Choudhary MI, Basha FZ, Abbas G, Khan SN, Shah S. Science at the interface of chemistry and biology: Discoveries of α-glucosidase inhibitors and antiglycation agents. Pure and Applied Chemistry. 2007;79(12):2263-8.
. Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986;232(4758):1629-32.
. Montagnani M. Diabetic cardiomyopathy: how much does it depend on AGE?. British journal of pharmacology. 2008;154(4):725-6.
. Choudhary MI, Abbas G, Ali S, Shuja S, Khalid N, Khan KM, Atta-ur-Rahman, Basha FZ. Substituted benzenediol Schiff bases as promising new anti-glycation agents. Journal of enzyme inhibition and medicinal chemistry. 2011;26(1):98-103.
. Whittier F, Spinowitz B, Wuerth JP. Pimagedine safety profile in patients with type 1 diabetes. J Am Soc Nephrol. 1999;10(9):184A.
. Cervantes-Laurean D, Schramm DD, Jacobson EL, Halaweish I, Bruckner GG, Boissonneault GA. Inhibition of advanced glycation end product formation on collagen by rutin and its metabolites. The Journal of nutritional biochemistry. 2006;17(8):531-40.
. Wu CH, Yen GC. Inhibitory effect of naturally occurring flavonoids on the formation of advanced glycation endproducts. Journal of agricultural and food chemistry. 2005;53(8):3167-73.
. Mosihuzzman M, Naheed S, Hareem S, Talib S, Abbas G, Khan SN, Choudhary MI, Sener B, Tareen RB, Israr M. Studies on α-glucosidase inhibition and anti-glycation potential of Iris loczyi and Iris unguicularis. Life sciences. 2013;92(3):187-92.
. Matsuda H, Wang T, Managi H, Yoshikawa M. Structural requirements of flavonoids for inhibition of protein glycation and radical scavenging activities. Bioorganic & medicinal chemistry. 2003;11(24):5317-23.
. Duraisamy Y, Gaffney J, Slevin M, Smith CA, Williamson K, Ahmed N. Aminosalicylic acid reduces the antiproliferative effect of hyperglycaemia, advanced glycation endproducts and glycated basic fibroblast growth factor in cultured bovine aortic endothelial cells: comparison with aminoguanidine. InVascular Biochemistry 2003:143-153.
. Matsuura N, Aradate T, Sasaki C, Kojima H, Ohara M, Hasegawa J, Ubukata M. Screening System for the Maillard Reaction Inhibitor from Natural Product Extracts. Journal of health science. 2002;48(6):520-6.
. Nagasawa T, Tabata N, Ito Y, Nishizawa N, Aiba Y, Kitts DD. Inhibition of glycation reaction in tissue protein incubations by water soluble rutin derivative. Biochemistry of Diabetes and Atherosclerosis 2003;3-10.
. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. Journal of immunological methods. 1983;65(1-2):55-63.
. Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival: modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. Journal of immunological methods. 1986;89(2):271-7.
. Nakagawa T, Yokozawa T, Terasawa K, Shu S, Juneja LR. Protective activity of green tea against free radical-and glucose-mediated protein damage. Journal of Agricultural and Food Chemistry. 2002;50(8):2418-22.
. Lehman TD, Ortwerth BJ. Inhibitors of advanced glycation end product-associated protein cross-linking. Biochimica et Biophysica Acta (BBA)-Molecular Basis of Disease. 2001;1535(2):110-9.
. Liu GZ. Clinical studies of gossypol as a male contraceptive. Reproduction. 1987;5:189-92.
. Dodou K. Investigations on gossypol: past and present developments. Expert opinion on investigational drugs. 2005;14(11):1419-34.
. Wang X, Howell CP, Chen F, Yin J, Jiang Y. Gossypol-a polyphenolic compound from cotton plant. Advances in food and nutrition research. 2009;58:215-63.
. Chadha S, Sanyal SN, Kanwar U. Reversibility of the effects of gossypol acetic acid, an antispermatogenic/antifertility agent on the intestinal structure and functions of male albino rats. Research in Experimental Medicine. 1989;189(3):205-19.
. Kenar JA. Reaction chemistry of gossypol and its derivatives. Journal of the American Oil Chemists' Society. 2006;83(4):269-302.
. Waites GM, Wang C, Griffin PD. Gossypol: reasons for its failure to be accepted as a safe, reversible male antifertility drug. International journal of andrology. 1998 (1):8-12.
. Royer RE, Deck LM, Campos NM, Hunsaker LA, Vander Jagt DL. Biologically active derivatives of gossypol: synthesis and antimalarial activities of peri-acylated gossylic nitriles. Journal of medicinal chemistry. 1986;29(9):1799-801.
. Baram NI, Ismailov AI. Biological activity of gossypol and its derivatives. Chemistry of Natural Compounds. 1993;29(3):275-87.
. Tukfatullina II, Tilyabaev KZ, Mamadrakhimov A, Salakhutdinov BA, Kamaev FG, Yuldashev AM, Dowd MK, Talipov SA, Ibragimov BT, Aripov TF. Membrane-active properties and antiradical activity of gossypol and its derivatives. Chemistry of natural compounds. 2008;44(4):440-5.
. Royer RE, Mills RG, Young SA, Vander Jagt DL. Comparison of the antiviral activities of 3′-azido-3′-deoxythymidine (AZT) and gossylic iminolactone (GIL) against clinical isolates of HIV-1. Pharmacological research. 1995;31(1):49-52.
. Royer RE, Deck LM, Vander Jagt TJ, Martinez FJ, Mills RG, Young SA, Vander Jagt DL. Synthesis and anti-HIV activity of 1, 1'-dideoxygossypol and related compounds. Journal of medicinal chemistry. 1995;38(13):2427-32.
. Mohanram I, Meshram J. Synthesis and biological activities of 4-aminoantipyrine derivatives derived from betti-type reaction. ISRN organic chemistry. 2014;2014.
. Ye K, Ke Y, Keshava N, Shanks J, Kapp JA, Tekmal RR, Petros J, Joshi HC. Opium alkaloid noscapine is an antitumor agent that arrests metaphase and induces apoptosis in dividing cells. Proceedings of the National Academy of Sciences. 1998;95(4):1601-6.
. Bulduk I, Taktak F. Isolation and characterization of antitumor alkaloid from poppy capsules (Papaver somniferum). Journal of Chemistry. 201213;2013.
. Moloudizargari M, Mikaili P, Aghajanshakeri S, Asghari MH, Shayegh J. Pharmacological and therapeutic effects of Peganum harmala and its main alkaloids. Pharmacognosy reviews. 2013;7(14):199.
. Sadykov AS, Otargaliev TO, Ishbaev AI, Abduvakhabov AA. Syntheses and investigations based on aphyllinic acid. Russian Chemical Bulletin. 1983;32(11):2332-7.
. Tilyabaev Z, Abduvakhabov AA. Alkaloids ofAnabasis aphylla and their cholinergic activities. Chemistry of natural compounds. 1998;34(3):295-7.
. Bunsupa S, Yamazaki M, Saito K. Quinolizidine alkaloid biosynthesis: recent advances and future prospects. Frontiers in plant science. 2012;3.
. Tojiboev A, Wang R, Pan F, Englert U, Turgunov K, Okmanov R. Insight into the chemical bonding and electrostatic potential: A charge density study on a quinazoline derivative. Journal of Structural Chemistry. 2013;54(6):1012-7.
. Gusarova NK, Malysheva SF, Oparina LA, Belogorlova NA, Tantsyrev AP, Parshina LN, Sukhov BG, Tlegenov RT, Trofimov BA. Synthesis of novel alkaloid derivatives from the vinyl ether of lupinine and PH-compounds. Arkivoc. 2009;7:260-7.
. Kulakov IV, and Nurkenov OA. Synthesis and Biological Activity of the Derivatives of Alkaloid Cytisine. Chemistry for Sustainable Development, 2012;20:237-250
. Kreft S, Knapp M, Kreft I. Extraction of rutin from buckwheat (Fagopyrum esculentum Moench) seeds and determination by capillary electrophoresis. Journal of agricultural and food chemistry. 1999;47(11):4649-52.