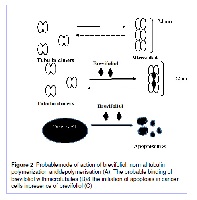
Brevifoliol: An Ignored cousin of Taxol
Keywords:
Brevifoliol, anticancer, Taxus, taxoid, Cancer, TaxolAbstract
The discovery of paclitaxel, an anticancer agent was a milestone in the path of anticancer drug discovery. After approval from FDA taxol was widely used for cancer treatment. The taxol was isolated from the bark of fully grown taxus plants with is a fatal source, to overcome this problem almost all the species were investigated for the taxol and taxol like molecules. Brevifoliol is one of the many taxoid isolated from the taxus plants. The significance of brevifoliol was its source which was dried needles of plants including the Himalayan yew tree Taxus wallichiana. Brevifoliol belongs to the large group of diterpenoid cyclodecanes, the same group with which taxol belongs. In-vitro studies indicate that brevifoliol has significant activity against colon cancer cell line which is slightly better than taxol. In the present article, all the updated information and its superiority over taxol will be discussed.
References
Appendino G, Barboni L, Gariboldi P, Bombordelli E, Gabetta B, Viterbo D (1993) Revised structure of brevifoliol and some baccatin VI derivatives. Journal of the Chemical Society, Chemical Communications 33: 1587–1589 Balza F, Tachibana S, Barrios H, Towers GHN (1991) Brevifoliol, a taxane from Taxus brevifolia. Phytochemistry 30:1613–1614 Baloglu E & Kingston DGI (1999) The taxane diterpenoids. Journal of natural products 62:1448–1472 Chattopadhyay SK, Tripathi S, Darokar MP et al (2008) Syntheses and cytotoxicities of the analogues of the taxoid brevifoliol. European journal of medicinal chemistry 43:1499–1505 Chordia MD, David GK, Ernest Hamel, et al (1997). Synthesis and biological activity of A-nor-paclitaxel analogues." Bioorganic & medicinal chemistry 5(5): 941-947 Chu A, Furlan M, Davin LB et al (1994). Phenylbutanoid and taxane-like metabolites from needles of Taxus brevifolia. Phytochemistry, 36(4), 975-985 Georg GI, Cheruvallath ZS & Velde DV et al (1993) Semisynthesis and biological evaluation of brevifoliol 13-[N-benzoyl-(-2′ R, 3′ S)-3′-phenylisoserinate]. Bioorganic & Medicinal Chemistry Letters 3:1349-1350. Georg GI, Gollapudi SR & Grunewald GL et al (1993a) A reinvestigation of the taxol content of Himalayan Taxus wallichiana Zucc and a revision of the structure of brevifoliol. Bioorganic & Medicinal Chemistry Letters 3:1345–1348 Kaur R, Chattopadhyay SK & Chatterjee A et al (2014). Synthesis and in vitro anticancer activity of brevifoliol derivatives substantiated by in silico approach. Medicinal Chemistry Research, 23(9), 4138-4148. Khanuja SPS, Santha Kumar TR, Garg A, Misra MK, Chattopadhyay SK, Srivastva S, Negi AK, United States Patent Publication No. 20040127561 A1, 2002. Kelland, LR, & Abel G. (1992). Comparative in vitro cytotoxicity of taxol and Taxotere against cisplatin-sensitive and-resistant human ovarian carcinoma cell lines. Cancer chemotherapy and pharmacology, 30(6), 444-450. Oberlies NH & Kroll DJ (2004) Camptothecin and taxol: historic achievements in natural products research Journal of natural products 67:129–135 Ramalingam S & Belani CP (2004) Paclitaxel for non-small cell lung cancer. Expert opinion on pharmacotherapy 5:1771–1780 Shi QW, Zhao YM & Si XT et al. (2006) 1-Deoxypaclitaxel and abeo-taxoids from the seeds of Taxus m airei. Journal of natural products 69:280–283 Schiff PB & Horwitz SB (1980) Taxol stabilizes microtubules in mouse fibroblast cells. Proceedings of the National Academy of Sciences 77:1561–1565 S Schiff PB, Fant J & Horwitz SB (1979) Promotion of microtubule assembly in vitro by taxol. Nature 277:665–667 Slichenmyer, WJ & Von Hoff DD. (1991). Taxol: a new and effective anti-cancer drug. Anti-Cancer Drugs, 2(6), 519-530 Tremblay S, Soucy C, Towers N et al (2004) Characterization of an abeo-Taxane: Brevifoliol and Derivatives. Journal of natural products, 67(5), 838-845. Walker FE. (1993) Paclitaxel (TAXOL®): Side Effects and Patient Education Issues. In Seminars in oncology nursing (Vol. 9, No. 4, pp. 6-10). WB Saunders. Wani MC, Taylor HL, Wall ME, et al. (1971) Plant antitumor agents. VI. The isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. Journal of the American Chemical Society 93:2325–2327 Zhao Y, Guo N, Lou LG, et al. (2008) Synthesis, cytotoxic activity, and SAR analysis of the derivatives of taxchinin A and brevifoliol. Bioorganic & medicinal chemistry 16:4860–4871