Antitesticular activities of different solvent fractions from hydro-methanol (2:3) extract of Cuminum cyminum in albino rat: A Comparative analysis
Keywords:
Hypotesticular activity, Apoptosis, Cuminum cyminum, Androgenesis, Oxidative stress, SpermatogenesisAbstract
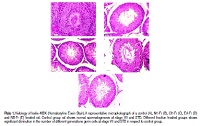
Currently available contraceptives are associated with adverse effects. So, search on safer agents in this purpose is one of the priority areas of WHO. Our previous study showed a significant antifertility effect of hydro-methanol extract of Cuminum cyminum Linn (Umbelliferae) in male albino rat. The main objective of this work isto search outthe potentfraction of hydro-methanol extract of seed of Cuminum cyminum in adult male albino rat for the development of herbal male contraceptive to reduce the bio-burden of phytomolecules. The n-hexane, chloroform, ethyl acetate, and n-butanol fractions of the hydro-methanol (2:3) extract of seed of Cuminum cyminum were administrated orally to male rat. Results showed the maximum antitesticular activity of chloroform fraction (CH-Fr) than other fractions included here. Treatment with CH-Fr fraction resulted a significant inhibition in spermiological parameters, activities of testicular androgenic key enzymes and antioxidative enzymes, levels of serum testosterone and seminal vesicular fructose, number of different generations of germ cells at stage VII of spermatogenic cell cycle and seminiferous tubular diameter (STD) along with significant increase in the level of testicular cholesterol in respect to the control. Significant upward and downward expression in Bax and Bcl-2 gene of male germ cells were indicated which focussed the sperm apoptotic enhancer activities of the fraction. The findings indicated that among the said four different fractions, the chloroform fraction of the hydro-methanol extract of the seed of Cuminum cyminum had most effective antitesticular activity.
References
Sheeja E, Joshi SB, Jain DC. Antiovulatory and estrogenic activity of Plumbago rosea leaves in female albino rats. Ind J Pharmacol. 2009;41:273–277.
Farnsworth NR, Bingel AS, Cordell GA, Crane FA, Fong HH. Potential value of plants as sources of new antifertility agents I. J Pharm Sci 1975;64:535–598.
Yakubu M, Akanji M, Oladiji A. Evaluation of antiandrogenic potentials of aqueous extract of Chromolaena odoratum (L.) KR leaves in male rats. Andrologia. 2007;39:235–243.
Goonasekera M, Gunawardana V, Jayasena K, Mohammed S, Balasubramaniam S. Pregnancy terminating effect of Jatropha curcas in rats. J Ethnopharmacol .1995;47:117–123.
Hoffer AP, Agarwal A, Meltzer P, Naqvi R, Matlin SA. Antifertility, spermicidal and ultrastructural effects of gossypol and derivatives administered orally and by intratesticular injections. Contraception .1988;37:301–331.
Chattopadhyay D, Dungdung S, Mandal A, Majumder G. A potent sperm motility-inhibiting activity of bioflavonoids from an ethnomedicine of Onge, Alstonia macrophylla Wall ex A. DC, leaf extract. Contraception. 2005;71:372–378.
Sandhyakumary K, Bobby RG, Indira M. Antifertility effects of Ricinus communis (Linn) on rats. Phyther Res. 2003;17:508–511.
Paul D, Bera S, Jana D, Maiti R, Ghosh D. In vitro determination of the contraceptive spermicidal activity of a composite extract of Achyranthes aspera and Stephania hernandifolia on human semen. Contraception. 2006;73:284–288.
Dhandapani S, Subramanian VR, Rajagopal S, Namasivayam N. Hypolipidemic effect of Cuminum cyminum L. on alloxan-induced diabetic rats. Pharmacol Res. 2002;46:251–255.
Jagtap AG, Patil PB. Antihyperglycemic activity and inhibition of advanced glycation end product formation by Cuminum cyminum in streptozotocin induced diabetic rats. Food Chem Toxicol. 2010;48:2030–2036.
Garg S. Antifertility screening of plants--effect of four indigenous plants on early pregnancy in female albino rats. Ind J Med Res .1976;64:1133–1135.
Chaudhury R, Haq M. Review of plants screened for antifertility activity-I. Bull Med Ethnobot Res .1980;1:408–419.
Gupta RS, Saxena P, Gupta R, Kachhawa JBS. Evaluation of reversible contraceptive activities of Cuminum cyminum in male albino rats. Contraception .2011;84:98–107.
Pakhira B, Jana K, Ghosh A, Ghosh D. Antitesticular activity of hydro-methanol extract of Cuminum cyminum in adult rat: a dose dependent study. Int J Pharm Pharm Sci .2015;7:285–291.
WHO. Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. 4th ed. New York: Cambridge University Press. 1999; 68-70.
Paul D, Mallick C, Ali KM, Nandi DK, Ghosh D. Duration dependent effect of hydro-ethanolic extract of leaf of S. hernandifolia and root of A. aspera on testicular androgenic and gametogenic activity: An approach for male herbal contraceptive development. Int J Appl Res Nat Prod. 2009;2:1–10.
Chatterjee K, Ali KM, De D, Mallick C, Ghosh D. Antihyperglycaemic, antioxidative activities of a formulated polyherbal drug MTEC (modified) in streptozotocin-induced diabetic rat. J Med Plants Res. 2009;3:468-80.
Meena R, Misro MM, Ghosh D, Nandan D. Complete sperm suppression induced by dienogest plus testosterone undecanoate is associated with down-regulation in the expression of upstream steroidogenic enzyme genes in rat testis. Contraception. 2012;86:163–171.
Maheshwari A, Misro MM, Aggarwal A, Sharma RK, Nandan D. Pathways involved in testicular germ cell apoptosis induced by H2O2 in vitro. FEBS J .2009;276:870–881.
Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–573.
Sokal R, Rohle F. Biometry. In: Sokal R, Rohle F, editors. Biometry, New York: WH Freeman and Company. 1997;179–206.
US Enviromental Pollution Agency. Health effects test guidelines OPPTS 870.3800. Reproduction and fertility effect. U.S. Enviromental Pollution Agency. 1998;1-12.
Patil S, Patil S, Londonkar R. Effect of pethidine on spermatogenesis in albino rats . Ind J Pharmacol .1998;30:249–253.
McLachlan RI, O’Donnell L, Meachem SJ, Stanton PG, de Kretser DM, Pratis K, et al. Identification of specific sites of hormonal regulation in spermatogenesis in rats, monkeys, and man. Recent Prog Horm Res. 2002;57:149–179.
Maeir DM. Species variation in testicular delta 5-3 beta-hydroxysteroid dehydrogenase activity: absence of activity in primate leydig cells. Endocrinol .1965;76:463–469.
Morris M, Chaikoff I. The origin of cholesterol in liver, small intestine, adrenal gland, and testis of the rat: dietary versus endogenous contributions. J Biol Chem. 1959;234:1095–1097.
Matsuoka T, Imai H, Asakuma S. Changes of fructose concentrations in seminal plasma and glucose and testosterone concentrations in blood plasma in rams over the course of a year. J Reprod Develop .2006;52:805-810.
Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Sci.1998;281:1322–1326.
Knudson CM, Tung KS, Tourtellotte WG, Brown GA, Korsmeyer SJ. Bax-deficient mice with lymphoid hyperplasia and male germ cell death. Sci .1995;270:96–99.