Cardioprotective Potential of Methanol Extract of Polygonum glabrum on Isoproterenol Induced Myocardial Necrosis in Rats
Keywords:
Polygonum glabrum, Isoproterenol, Necrosis, Marker enzymesAbstract
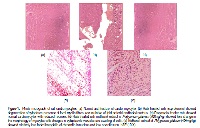
Aim: The aim of present study was to evaluate the cardioprotective efficacy of Polygonum glabrum on isoproterenol induced myocardial necrosis in rats. Methods: Experimental rats were treated orally with methanol extract of Polygonum glabrum at two doses (200 mg and 400 mg/kg) for 30 days. Isoproterenol (85 mg/kg, s.c.) was administered on 29th and 30th day to induce myocardial necrosis. At the end of the experiment, serum cardiac marker enzymes [creatine kinase muscle brain (CK-MB), lactate dehydrogenase (LDH), serum glutamate oxaloacetate transaminase (SGOT)], serum glutamate pyruvate transaminase (SGPT) and total protein (TP) were estimated. Plasma total cholesterol (TC), triglycerides (TG), high density lipoprotein (HDL), low density lipoprotein (LDL) and very low density lipoprotein (VLDL) levels were also recorded. Further, antioxidant parameters viz catalase (CAT), superoxide dismutase (SOD), glutathione (GSH), glutathione peroxidase (GPx), glutathione reductase (GRD) and malondialdehyde (MDA) levels were evaluated in heart tissue homogenate. Results: The results of the study indicated that, methanol extract of Polygonum glabrum showed greater cardioprotection by restoring the cardiac marker enzymes and attenuated the level of plasma lipid profiles along with an increase in HDL. Additionally, level of myocardial antioxidants significantly increased along with a reduction in the content of malondialdehyde. The cardioprotective effect was compared with propranolol (10 mg/kg, oral) which was used as the standard. Histopathological findings revealed a decrease in the degree of necrosis and inflammation following pretreatment with Polygonum glabrum. Conclusion: The present investigation indicates that Polygonum glabrum could protect myocardium from isoproterenol induced necrosis.
References
Bono DP, Boon NA. In: Davidson’s Principles and Practice of Medicine. Diseases of cardiovascular system, Edwards CRW, Boucheir IA, editors. Hong Kong. 1992;249–340.
. Farvin KH, Anandan R, Kumar SH, Shiny KS, Sankar TV, Nair PG. Protective effect of squalene on changes in lipid profile in experimentally induced myocardial infarction in rats. J. Med. Food. 2006;9(4):531–6.
. Adaramoye OA, Medeiros IA. Endothelium independent vasodilation induced by kolaviron, a biflavonoid complex from Garcinia kola seeds, in rat superior mesenteric arteries. J. Smooth. Muscle. Res. 2009; 45(1):39–53.
. Adaramoye OA, Lawal SO. Kolaviron, a biflavonoid fraction from Garcinia kola, protects against isoproterenol-induced injury by mitigating cardiac dysfunction and oxidative stress in rats. J. Basic. Clin. Physiol. Pharmacol. 2015; 26(1):65-72.
. Ahmad B, Rehman MU, Amin I, Arif A, Rasool S, Bhat SA, Afzal I, Hussain I, Bilal S, Mir M. A Review on Pharmacological properties of zinger one (4-(4-Hydroxy-3methoxyphenyl)-2-butanone). Scientific. World. J. 2015; 816364.
. Upaganlawar A, Gandhi H, Balaraman R. Isoproterenol induced myocardial infarction: Protective role of natural products. J. Pharmacol. Toxicol. 2011; 6: 1-17.
. Alcantara EH, Shin MY, Sohn HY, Park YM, Kim T, Lim JH, Jeong HJ, Kwon ST, Kwun IS. Diosgenin stimulates osteogenic activity by increasing bone matrix protein synthesis and bone-specific transcription factor Runx2 in osteoblastic MC3T3-E1 cells. J. Nutr. Biochem. 2011; 22(11):1055-1063.
. Bafna PA, Balaraman R. Antioxidant activity of DHC-1, a herbal formulation, in experimentally-induced cardiac and renal damage. Phytother Res. 2005; 19(3): 216–222.
. Shankar LH, Mishra PK. Study of aquatic medicinal plants of Hazaribagh district of Jharkhand,India. Int. Res. J. pharm. 2012; 3(4): 405-409.
. Khan MF, Islam Rabbi SN, Fahima Aktar MD, Kawsar H. In vitro cytotoxic, membrane stabilizing and thrombolytic activities of Polygonum glabrum Willd. Bangladesh. pharm. j. 2014; 17(2): 202-204.
. Babitha S, David B, Otilia JFB. Investigation on antioxidant and hepatoprotective activity of ethanolic leaf extract of Polygonum glabrum Wild on carbon tetrachloride- induced hepatotoxicity in rats. Spatula DD. 2012; 2(4): 199-205.
. Palani R, Karunakaran D, Rajesh V, Mathivanan K ,Jayaraman P. Analysis of antioxidant, antimicrobial activity and phytochemical potential of Cleistanthus collinus Roxb.,Polygonum glabrum Wild. and Melia azedarch Linn. Asian J. Med. Pharm. Sci. 2014; 2(2):149-159.
. Said MS, Chinchansure AA, Nawale L, Durge A, Wadhwani A, Kulkarni SS, Sarkar D, Joshi SP. A new butenolide cinnamate and other biological active chemical constituents from Polygonum glabrum. Nat. Prod. Res. 2015;29(22): 2080-2086,
. Mohammed IS. Phytochemical studies of flavonoids from Polygonum glabrum L. of Sudan. MSc thesis, Chemistry, University of Khartoum.1996.
. Jacobsson U, Muddathir AK. Four biologically active Sesquiterpenes of the drimane type isolated from Polygonum glabrum. Phytochemistry. 1992; 31(12):4207–4211.
. Chandan BK, Saxena AK, Shukla S, Sharma N, Gupta DK, Suri KA, Suri J, Bhadauria M, Singh B. Hepatoprotective potential of Aloe barbadensis mill against carbon tetrachloride induced hepatotoxicity. J Ethnopharmacol. 2007; 111(3): 560–6.
. Reitman S, Frankel SA. Colourimetric method for the determination of serum oxaloacetatic and glutamic pyruvic transaminases. Amer J Clin Pathol. 1957; 28(1): 56–63.
. Kornberg A. Lactate dehydrogenase of muscle. In, SP Colowick, NO Kaplan (Eds.) Methods in Enzymology. Academic Press, New York. 1955: 441–443.
. Lowry OH, Rosebrough NJ, Farr AL, and Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951; 193(1): 265–275.
. Aebi H. Catalase. Methods in enzymatic analysis. H.V. Bergmeyer. New York, Cheime, Weinheim, FRG: Academic press. 1974; 2: 674–684.
. Rai S, Wahile A, Mukherjee K, Saha BP, Mukherjee PK. Antioxidant activity of Nelumbo nucifera (sacred lotus) seeds. J Ethnopharmacol.2006;104(3):322-327.
. Ellman GL. Tissue sulfhydryl groups. Arch. Biochem. Biophysic. 1959; 82(1):70–77
. Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller D. Differential distribution of glutathione and glutathione related enzymes in rabbit kidney: possible interactions in analgesic neuropathy. Biochem Pharmacol. 1984;33(11):1801-7
. Wendel A. Glutathione peroxidase. Methods in Enzmology. 1981; 77: 325–33.
. Liu JR. Edamatsu H, Kabuto, Mori A. Antioxidant action of Guilingji in the brain of rats with FeCl3 induced epilepsy. Free Radical Biology and Medicine. 1990; 9: 451–54.
. Deodato B, Altavilla D, Squadrito G,Compo G M ,Arlottan M, Mirutoli L,et al. Cardio protection by the phytoestrogengenistein in experimental myocardial ischemia-reperfusion injury. Br J Pharmacol 1999;128(8):1683–90.
. Robbins SL. Cell Injury, Cell Death, and Adaptations. Robbins pathologic basis of disease. 8th ed. chapter 2 Philadelphia, PA : Saunders/Elsevier; 2007.
. Priscilla DH, Prince PSM. Cardioprotective effect of gallic acid on cardiac troponin-T, cardiac marker enzymes, lipid peroxidation products and antioxidants in experimentally induced myocardial infarction in Wistar rats. Chem. Biol. Interact. 2009; 179(2-3):118–124.
. Dhavan S, Kapoor NK, Nityanand S. Effect of isoprenaline on lipid profile and cardiac enzymes in rats. Ind. J. Exp. Biol. 1978;16(3):376–8.
. Dimon GS, Gerbaud P, Keryer G, Anderson W, Evain BD, Raynaud FJ. In vitro effects of oxygen-derived free radicals on type I and type II cAMP-dependent protein kinases. Biol Chem. 1998; 273(35):22833-40.
. Radhika S, Smila KH, Muthezhilan R Cardioprotective activity of Hybanthus enneaspermus (linn.) on isoproterenol induced Rats. Ind. J. Fund. Appl. Life Sci 2011;1(3): 90‒97.
. Somova LO, Nadar A, Rammanan P, Shode FO. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003; 10(2-3): 115-121.
. Kang YJ, Sun X, Chen Y, Zhou Z.Inhibition of doxorubicin chronic toxicity in catalase-overexpressing transgenic mouse hearts. Chem. Res. Toxicol. 2002;15(1):1-6
. Nirmala C, Puvanakrishnan R. Protective role of curcumin against isoproterenol induced myocardial infarction in rats. Mol. Cell. Biochem. 1996;159(2):85–93.
. Meister A. Glutathione metabolism and its selective modification. J.Biol.Chem.1988; 263(33):17205–8.
. Thounaojam, MC Jadeja, RN Ansarullah, SS Karn, JD Shah, DK Patel, et al. Cardioprotective effect of Sida rhomboida. Roxb extract against isoproterenol induced myocardial necrosis in rats. Exp. Toxicol. Pathol. 2010; 63:351-356.