Phytochemical screening and influence of extraction solvents on antioxidant and antimicrobial activities of Asparagus racemosus willd. Root
Keywords:
Asparagus racemosus, Phytochemical compounds, Antioxidant activities, Antimicrobial activities, extraction solventAbstract
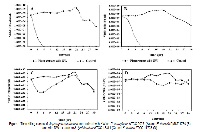
The bioactive components present in the Asparagus racemosus root are known to be responsible for its medicinal properties. However, the solvent extraction for these bioactive components had effect on bioactive activities. The present study was aimed to compare the effect of using different extraction solvents to extract the active components, antioxidant and antimicrobial activities from Asparagus racemosus root. Aqueous (DW and NW) extracts showed the efficiency of Asparagus racemosus root as antioxidant agent and broad spectrum antibacterial agent against both Gram-positive and Gram-negative. 95% Ethanolic extract had the most potential in the Ferric Reducing Antioxidant Power (FRAP) of 14.400±0.001 mg AAE/100g FW. But aqueous (DW and NW) extracts showed the lowest IC50 value of 4.716±0.002 - 4.757±0.001 mg/ml (IC50 ascorbic acid of 3.422±0.001 mg/ml). Moreover, Natural distilled water extract had also the bactericidal effect on P. aeruginasa ATCC 27853 and K. pneumoniae and E. faecalis DMST 4736 at 3-6 h intervals after incubation. These biological activity may be due partly to the presence of various phytochemical compounds; phenolics, flavonoids saponins, steroids, terpenoids and cardiac glycosides. It also suggests that Asparagus racemosus root was a potential candidate in antioxidant and antimicrobial agents. It was useful in applications with modern medicine in the therapy or prevention of disease as well as the adoption in commercial various health products in the future. In addition to the ethanol and water were suitable solvent to extract the substance, natural distilled water was also the alternative solvent for bioactive compounds extraction.
References
. Bushra S, Farooq A, Muhammad A. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009;14(6):2167- 2180. [2]. Bopana N, Saxena S. Asparagus racemosus : Ethnopharmacological evaluation and conservation needs. J Ethnopharmacol 2007;110(1):1-15. [3]. Velavan S, Begum HV. Restorative effect of Asparagus racemosus on agerelated oxidative damage in heart lysosome of aged rats. Int J Pharmacol 2007;3(1):48-54. [4]. Chawla A, Chawla P, Mangalesh P, Roy RC. Asparagus racemosus (Willd): Biological Activities & its Active Principles. Indo Global J Pharm Sci 2011;1(2):113-120. [5]. Bhatnagar M, Sisodia SS, Bhatnagar R. Antiulcer and antioxidant activity of Asparagus racemosus Willd. and Withania somnifera Dunal in rats. Ann N Y Acad Sci 2005;1056:261–278. [6]. Battu GR, Kumar BM. Anti-inflammatory activity of leaf extract of Asparagus racemosus Willd. Int J Chem Sci 2010;8(2):1329-1338. [7]. Madahavan V, Ranajit DT, Mythreyi R, Gurudeva MR, Yoganarasimhan SN. Pharmacolgnostical studies on the root tubers of Asparagus racemosus Baker- Alternative source for the Ayurvedic drug Shatavari. Indian J Nat Prod Resour 2010;1(1):57-62. [8]. Raval PK, Nishteshwar K, Patel BR, Shukla J. 2012. Asparagus racemosus Wild. – A Comparative Phytochemical analysis of fresh dried roots of Shatavari. Int j pharm biol sci arch 2012;3(6):1458-1461. [9]. Trease GE, Evans WC. Pharmacognosy. 15th ed, Edinburgh (New York): WB Saunders; 2002. [10]. Harborne JB. Phytochemical Methods. A Guide to Modern Techniques of Plant Analysis. 3rd ed. London: Chapman & Hall; 1998. p. 182-190. [11]. Materska M, Perucka I. Antioxidant activity of the main phenolic compounds Isolated from Hot pepperfruit (Capsicum annuum L.). J Agric Food Chem 2005;53(5):1750-1756. [12]. Marinova D, Ribarova F, Atanassova M. Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Chem Technol Metall 2005;40(3):255-260. [13]. Akowuah GA, Ismail Z, Norhayati I, Sadikun A. The effects of different extraction solvents of varying polarities of polyphenols of Orthosiphon stamineus and evaluation of the free radical-scavenging activity. Food Chem 2005;93(2):311-317. [14]. Benzie IFF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of ‘‘antioxidant power’’: The FRAP assay. Anal Biochem 1996;239:70–76. [15]. Irshad S, Maryum M, Farzana P. In vitro antibacterial activities of three medicinal plants using agar well diffusion method. Res J Biol 2012;2(1):1-8. [16]. National Committee for Clinical Laboratory Standards. Methods for determining bactericidal activity of antimicrobial agents, Approved guideline M26-A. Wayne, PA: NCCLS; 1999. [17]. May J, Chan CH, King A, Williams L, French GL. Time-kill studies of tea tree oils on clinical isolates. J Antimicrob Chemother 2000;45(5):639-643. [18]. Polterai O. Antioxidants and free-radical scavengers of Natural Origin. Curr Org Chem 1997;1(4):415–440. [19]. Augusto LS, Josean FT, Marcelo S, Margareth FM, Petronio FA, Jose, MB 2011. Anti-inflammatory activity of alkaloids: an update from 2000 to 2010. Molecules 2011;16(10): 8515–8534. [20]. Dua VK, Gaurav V, Bikram S, Aswathy R, Upma B, Dau DA, et al. Anti-malarial property of steroidal alkaloid conessine isolated from the bark of Holarrhena antidysenterica. Malar J 2013;12(1):1–6. [21]. Benbott A, Yahyia A, Belaïdi A. 2012. Assessment of the antibacterial activity of crude alkaloids extracted from seeds and roots of the plant Peganum harmala L. J Nat Prod Plant Resour 2012;2(5):568–573. [22]. Ameyaw Y, Duker-Eshun G. The alkaloid contents of the ethno-plant organs of three antimalarial medicinal plant species in the eastern region of Ghana. Int J Chem Pet Sci 2009;7(1):48–58. [23]. Thite SV, Chavan YR, Aparadh VT, Kore BA. Preliminary phytochemical screening of some medicinal plants. Int J Pharm Chem Biol Sci 2013;3(1):87–90. [24]. Alexei YB, Joseph IS, Olga VF. Endogenous cardiotonic steroids: physiology, pharmacology and novel therapeutic targets. Pharmacol Rev 2009;61(1):9–38. [25]. Vladimir K, Ludmila M. Glycosides in medicine: the role of glycosidic residue in biological activity. Curr Med Chem 2001;8(11):1303–1328. [26]. Ayoola AG, Ipav SS, Sofidiya MO, Adepoju-Bello AA, Coker AB, Odugbemi TO. Phytochemical screening and free radical scavenging activities of the fruits and leaves of Allanblackia floribunda Oliv (Guttiferae). Int J Health Res 2008;1(2):87-93. [27]. Rajanandh MG, Kavitha J. Quantitative estimation of β-sitosterol, total phenolic and flavonoid compounds in the leaves of Moringa oleifera. Int J PharmTech Res 2010;2(2):1409–1414. [28]. Maisuthisakul P, Suttajit M, Pongsawatmanit R. Assessment of phenolic content and free radical scavenging capacity of some Thai indigenous plants. Food Chem 2007;100(4): 1409-1418. [29]. Gan RY, Xu XR, Song FL, Kuang L, Li HB. Antioxidant activity and total phenolic content of medicinal plants associated with prevention and treatment of cardiovascular and cerebrovascular diseases. J Med Plants Res 2010;4(22):2438–2444. [30]. Cai YZ, Sun M, Corke H. Antioxidant activity of betalains from plants of the Amaranthaceae. J Agric Food Chem 2003;51(8):2288–2294. [31]. Moure A, Cruz JM, Franco D, Domínguez JM, Sineiro J, Domínguez H, et al. Natural antioxidants from residual sources. Food Chem 2001;72(1):145-171.