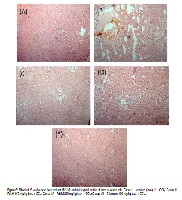
Ameliorative effect of Ficus dalhousiae Miq. (Moraceae) methanolic leaf extract on carbon tetrachloride induced hepatic and renal toxicity
Keywords:
superoxide dismutase, catalase, glutathione and reactive oxygen speciesAbstract
Developing traditional medicine in the field of hepatology and nephrology research is the key to pharmacology. This study demonstrates the mechanism of hepatoprotective and renal protective activity of Ficus dalhousiae Miq. (Moraceae) methanolic leaf extract on carbon tetrachloride induced hepatic and renal toxicity. Shade dried powder was subjected to shoxlet extraction with methanol and assessed for hepatoprotective and renal protective activities. Hepatotoxicity and renal toxicity were induced in rats by single oral dose of CCl4 diluted with olive oil (1:1 v/v; ml/kg body weight) after pretreatment of methanolic extract for seven days. Sixteen hrs after CCl4 administration, rats were sacrificed and biochemical markers like Serum alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) estimated followed by the measurement of liver and kidney cytosolic antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione (GSH) and reactive oxygen species (ROS). The data were analysed by one way analysis of variance (ANOVA). The extract at the doses of 150 and mg/kg b.w. significantly reduces elevated levels of ALT, AST, ALP and LDH. The extracts also showed significant increase in the reduced level of SOD, CAT and GSH. The ROS activity also found down regulated. The activity of methanolic extracts were comparable with the standard Silymarin. These findings not only showed potential hepatoprotective and renal protective activities of Ficus dalhousiae but also manifested by restoring antioxidant enzymes. With this pilot study we can justify the medicinal importance of this plant.
References
. Sarkar K, Ghosh A, Kinter, M, Mazumder B, Sil PC. Purification and Characterization of a 43 kD Hepatoprotective Protein from the Herb Cajanus indicus L. The Protein Journal 2006; 25: 411-421.
. Abraham P, Wilfred G, Cathrine S. Oxidative damage to the lipids and proteins of the lungs, testis and kidney of rats during carbon tetrachloride intoxication. Clinica Chimica Acta. 1999; 289: 177-179.
. Szymonik‐Lesiuk S, Czechowska G, Stryjecka‐Zimmer M, SŁomka M, Mądro A, CeliŃski K, Wielosz M. Catalase, superoxide dismutase, and glutathione peroxidase activities in various rat tissues after carbon tetrachloride intoxication. Journal of hepato-biliary-pancreatic surgery. 2003; 10: 309-315.
. Güven A, Gülmez M. The Effect of Kefir on the Activities of GSH‐Px, GST, CAT, GSH and LPO Levels in Carbon Tetrachloride‐Induced Mice Tissues. Journal of Veterinary Medicine, Series B .2003; 50: 412-416.
. Charbonneau M, Brodeur J, du Souich P, Plaa GL. Correlation between acetone-potentiated CCl 4-induced liver injury and blood concentrations after inhalation or oral administration. Toxicology and applied pharmacology 1986; 84: 286-294.
. Ahmad F, Cowan D, Sun A. Detection of free radical formation in various tissues after acute carbon tetrachloride administration in gerbil. Life sciences.1987; 41: 2469-2475.
. Ohta Y, Nishida K, Sasaki E, Kongo M, Ishiguro I. Attenuation of disrupted hepatic active oxygen metabolism with the recovery of acute liver injury in rats intoxicated with carbon tetrachloride. Research communications in molecular pathology and pharmacology. 1997; 95: 191-207.
. Ozturk F, Ucar M, Ozturk IC, Vardi N, Batcioglu K. Carbon tetrachloride-induced nephrotoxicity and protective effect of betaine in Sprague-Dawley rats. Urology. 2003; 62: 353-356.
. Devasagayam T, Tilak J, Boloor K, Sane KS, Ghaskadbi SS, Lele R. Free radicals and antioxidants in human health: current status and future prospects. Japi. 2004; 52:4.
. Ko KM, Ip SP, Poon MKT, Wu S, Che CT, Ng KH, Kong YC. Effect of a lignan-enriched fructus schisandrae extract on hepatic glutathione status in rats: protection against carbon tetrachloride toxicity. Planta medica. 1995; 61: 134-137.
. Rajesh M, Latha M. Protective activity of Glycyrrhiza glabra Linn. on carbon tetrachloride-induced peroxidative damage. Indian Journal of Pharmacology. 2004; 36:284.
. Ghori SS, Khan M, Khan SA, Baig MD. Antihyperglycaemic Effect Of Ficus dalhousiae Miq Leaf Ethanolic Extract In Alloxan–Induced Diabetic Rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2014; 6: 132-136.
. Ghori SS, Khan M, Tabassum R. Anti-Inflammatory activity Of Ficus dalhousiae Miq roots ethanolic extract in Wistar Albino rats. Asian Journal of Pharmaceutical and Clinical Research. 2015; 8: 117-119.
. Kumara K, Sringeswara A, Sadananda K, Prakash H. New distribution record of the endemic and rare Ficus dalhousiae Miq.(Moraceae). Journal of Threatened Taxa. 2013; 5: 4808-4810.
. Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959; 82: 70-77.
. Aebi H. [13] Catalase in vitro. Methods in Enzymology. 1984; 105: 121-126.
. Habbu P, Shastry R, Mahadevan K, Joshi H, Das S. Hepatoprotective and antioxidant effects of Argyreia speciosa in rats. African Journal of Traditional, Complementary and Alternative Medicines. 2008; 5: 158-164.
. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. European Journal of Biochemistry. 1974; 47: 469-474.
. Cathcart R, Schwiers E, Ames BN. Detection of picomole levels of hydroperoxides using a fluorescent dichlorofluorescein assay. Analytical Biochemistry . 1983; 134: 111-116.
. Galighor A, Kozloff E. Essentials of practical microtechnique 2nd edn. Lea and Febiger, NewYork. 1976.
. Brattin WJ, Glende A, Recknage RO. Pathological mechanisms in carbon tetrachloride hepatotoxicity. Journal of Free Radicals in Biology & Medicine. 1985; 1: 27-38.
. Basu S. Carbon tetrachloride-induced lipid peroxidation: eicosanoid formation and their regulation by antioxidant nutrients. Toxicology. 2003; 189: 113-127.
. Barapatre A, Aadil KR, Tiwary BN, Jha H. In vitro antioxidant and antidiabetic activities of biomodified lignin from Acacia nilotica wood. International Journal of Biological Macromolecules. 2015; 75: 81-89.
. Barapatre A, Meena AS, Mekala S, Das A, Jha H. In vitro evaluation of antioxidant and cytotoxic activities of lignin fractions extracted from Acacia nilotica. International Journal of Biological Macromolecules. 2016; 86: 443-453.