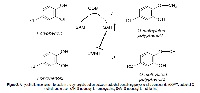
A supposed mechanism of synergistic action of catechol-containing natural polyphenols
Keywords:
catecholic phytochemicals, catechol-O-methyltransferase, chemoprevention, cytotoxicity, flavonoids, synergistic bioactivitiesAbstract
Over the past decades, accumulated evidences have been published about different synergistic biological activities between natural dietary polyphenols. Although these effects could be physiologically important in chemoprevention, cardioprotection and neuroprotection, but probably also in treatment of serious chronic diseases, such as cancer, the exact mechanisms behind this potentiation have still remained largely unknown. In this article, supposition about the involvement of phase II metabolic enzyme, catechol-O-methyltransferase (COMT), in the synergistic action of catechol-containing polyphenols is proposed. Serving as substrates, these compounds can also behave as COMT inhibitors suppressing the O-methylation of the other catechol-containing component in the combined mixture. At that, negative feedback by the increased amount of S-adenosyl-L-homocysteine generated from the methyl-group donor S-adenosyl-L-methionine during the enzymatic conversion can play an important role. Presuming that O-methylated conjugates are in general biologically less active than their unmetabolised counterparts, cotreatment of cells with combination of two catecholic natural agents can lead to a superior effect as compared to the administration of either compound alone. This mechanism can provide an explanation to the beneficial synergistic effects described for green tea extracts in chemoprevention or red wine consumption in protection of cardiovascular system in comparison with their single components tested separately. However, as currently only little is known about the possible biological activities of O-methylated conjugates of dietary polyphenolic phytochemicals, their nature and effects definitely need to be further studied. These results could prove (or disprove) the hypothesis raised in this article but also contribute to the development of physiologically or even clinically useful mixtures of polyphenols with catechol structure in the future.
References
. Amin AR, Wang D, Zhang H, et al. Enhanced anti-tumor activity by the combination of the natural compounds (-)-epigallocatechin-3-gallate and luteolin: potential role of p53. J Biol Chem 2010;285(45):34557-65.
. Xu R, Zhang Y, Ye X, et al. Inhibition effects and induction of apoptosis of flavonoids on the prostate cancer cell line PC-3 in vitro. Food Chem 2013;138(1):48-53.
. Pignatelli P, Pulcinelli FM, Celestini A, et al. The flavonoids quercetin and catechin synergistically inhibit platelet function by antagonizing the intracellular production of hydrogen peroxide. Am J Clin Nutr 2000;72(5):1150-5.
. Redondo A, Estrella N, Lorenzo AG, Cruzado M, Castro C. Quercetin and catechin synergistically inhibit angiotension II-induced redox-dependent signalling pathways in vascular smooth muscle cells from hypertensive rats. Free Radic Res 2012;46(5):619-27.
. Nichols M, Zhang J, Polster BM, et al. Synergistic neuroprotection by epicatechin and quercetin: Activation of convergent mitochondrial signaling pathways. Neuroscience 2015;308:75-94.
. Sak K. Cytotoxicity of dietary flavonoids on different human cancer types. Pharmacogn Rev 2014;8(16):122-46.
. Tang SN, Singh C, Nall D, Meeker D, Shankar S, Srivastava RK. The dietary bioflavonoid quercetin synergizes with epigallocatechin gallate (EGCG) to inhibit prostate cancer stem cell characteristics, invasion, migration and epithelial-mesenchymal transition. J Mol Signal 2010;5:14.
. Gray AL, Stephens CA, Bigelow RL, Coleman DT, Cardelli JA. The polyphenols (-)-epigallocatechin-3-gallate and luteolin synergistically inhibit TGF-β-induced myofibroblast phenotypes through RhoA and ERK inhibition. PLoS One 2014;9(10):e109208.
. Suganuma M, Okabe S, Kai Y, Sueoka N, Sueoka E, Fujiki H. Synergistic effects of (-)-epigallocatechin gallate with (-)-epicatechin, sulindac, or tamoxifen on cancer-preventive activity in the human lung cancer cell line PC-9. Cancer Res 1999;59(1):44-7.
. Shimizu M, Deguchi A, Lim JT, Moriwaki H, Kopelovich L, Weinstein IB. (-)-Epigallocatechin gallate and polyphenon E inhibit growth and activation of the epidermal growth factor receptor and human epidermal growth factor receptor-2 signaling pathways in human colon cancer cells. Clin Cancer Res 2005;11(7):2735-46.
. Park CM, Jin KS, Lee YW, Song YS. Luteolin and chicoric acid synergistically inhibited inflammatory responses via inactivation of PI3K-Akt pathway and impairment of NF-κB translocation in LPS stimulated RAW 264.7 cells. Eur J Pharmacol 2011;660(2-3):454-9.
. Sak K, Kasemaa K, Everaus H. Potentiation of luteolin cytotoxicity by flavonols fisetin and quercetin in human chronic lymphocytic leukemia cell lines. Food Funct 2016;7(9):3815-24. .
. Park CM, Park JY, Noh KH, Shin JH, Song YS. Taraxacum officinale Weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J Ethnopharmacol 2011;133(2):834-42.
. Zhu BT. Catechol-O-Methyltransferase (COMT)-mediated methylation metabolism of endogenous bioactive catechols and modulation by endobiotics and xenobiotics: importance in pathophysiology and pathogenesis. Curr Drug Metab 2002;3(3):321-49.
. Bai HW, Shim JY, Yu J, Zhu BT, Biochemical and molecular modeling studies of the O-methylation of various endogenous and exogenous catechol substrates catalyzed by recombinant human soluble and membrane-bound catechol-O-methyltransferases. Chem Res Toxicol 2007;20(10):1409-25.
. Zhu BT, Patel UK, Cai MX, Lee AJ, Conney AH. Rapid conversion of tea catechins to monomethylated products by rat liver cytosolic catchol-O-methyltransferase. Xenobiotica 2001:31(12):879-90.
. Chen Z, Zheng S, Li L, Jiang H. Metabolism of flavonoids in human: a comprehensive review. Curr Drug Metab 2014;15(1):48-61.
. Goodman JE, Lavigne JA, Wu K, et al. COMT genotype, micronutrients in the folate metabolic pathway and breast cancer risk. Carcinogenesis 2001;22(10):1661-5.
. Zhu BT, Ezell EL, Liehr JG. Catechol-O-methyltransferase-catalyzed rapid O-methylation of mutagenic flavonoids. Metabolic inactivation as a possible reason for their lack of carcinogenicity in vivo. J Biol Chem 1994;269(1):292-9.
. Zhu BT, Patel UK, Cai MX, Conney AH. O-Methylation of tea polyphenols catalyzed by human placental cytosolic catechol-O-methyltransferase. Drug Metab Dispos 2000;28(9):1024-30.
. Chen ZJ, Dai YQ, Kong SS, et al. Luteolin is a rare substrate of human catechol-O-methyltransferase favoring a para-methylation. Mol Nutr Food Res 2013;57(5):877-85.
. Forester SC, Lambert JD. Synergistic inhibition of lung cancer cell lines by (-)-epigallocatechin-3-gallate in combination with clinically used nitrocatechol inhibitors of catechol-O-methyltransferase. Carcinogenesis 2014;35(2):365-72.
. Fang MZ, Wang Y, Ai N, et al. Tea polyphenol (-)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res 2003;63(22):7563-70.
. Wang P, Aronson WJ, Huang M, et al. Green tea polyphenols and metabolites in prostatectomy tissue: implications for cancer prevention. Cancer Prev Res (Phila) 2010;3(8):985-93.
. Landis-Piwowar K, Chen D, Chan TH, Dou QP. Inhibition of catechol-O-methyltransferase activity in human breast cancer cells enhances the biological effect of the green tea polyphenol (-)-EGCG. Oncol Rep 2010;24(2):563-9.
. Zhu BT, Shim JY, Nagai M, Bai HW. Molecular modelling study of the mechanism of high-potency inhibition of human catechol-O-methyltransferase by (-)-epigallocatechin-3-O-gallate. Xenobiotica 2008;38(2):130-46.
. Zhu BT, Wu KY, Wang P, Cai MX, Conney AH. O-methylation of catechol estrogens by human placental catechol-O-methyltransferase: interindividual differences in sensitivity to heat inactivation and to inhibition by dietary polyphenols. Drug Metab Dispos 2010;38(10):1892-9.
. Zhu BT, Liehr JG. Quercetin increases the severity of estradiol-induced tumorigenesis in hamster kidney. Toxicol Appl Pharmacol 1994;125(1):149-58.
. Zhu BT, Liehr JG. Inhibition of catechol O-methyltransferase-catalyzed O-methylation of 2- and 4-hydroxyestradiol by quercetin. Possible role in estradiol-induced tumorigenesis. J Biol Chem 1996;271(3):1357-63.
. Nagai M, Conney AH, Zhu BT. Strong inhibitory effects of common tea catechins and bioflavonoids on the O-methylation of catechol estrogens catalyzed by human liver cytosolic catechol-O-methyltransferase. Drug Metab Dispos 2004;32(5):497-504.
. Wang P, Heber D, Henning SM. Quercetin increased bioavailability and decreased methylation of green tea polyphenols in vitro and in vivo. Food Funct 2012;3(6):635-42.
. Wang P, Heber D, Henning SM. Quercetin increased the antiproliferative activity of green tea polyphenol (-)-epigallocatechin gallate in prostate cancer cells. Nutr Cancer 2012;64(4):580-7.
. Bode AM, Dong Z. Epigallocatechin 3-gallate and green tea catechins: United they work, divided they fail. Cancer Prev Res (Phila) 2009;2(6):514-7.