Anti Urolithiatic and anti hyperlipidemic activity of Coleus aromaticus An explanation of the underlying mechanisms
Keywords:
Coleus aromaticus, Calcium oxalate crystals, Hypolipidemic activity, Antioxidant activityAbstract
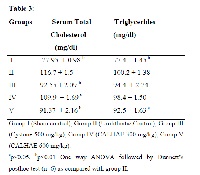
Leaves paste of Coleus aromaticus is used as a traditional remedy for urolithiasis in India. In the present study, the anti urolithiatic activity of Coleus aromaticus was investigated in ethylene glycol induced urolithiatic rats. There was a significant increase in the levels of calcium oxalate crystals in the kidneys as well as lipid levels in the blood serum. Treatment with hydro alcoholic extract of C.aromaticus leaves (CALHAE) significantly reduced cholesterol levels at 300 and 600 mg/kg, and triglyceride levels at 600 mg/kg in urolithiatic rats. Histopathalogical reports confirmed that chronic administration of CALHAE (300 and 600 mg/kg) diminished number of calcium oxalate crystals in kidneys. CALHAE has shown reduction in thiobarbituric acid reactive substances (TBARS) in urolithiatic rats. Moreover, CALHAE showed potent in vitro antioxidant activity in DMPD, ABTS radicals (MnO2 method). Results from these studies support the safe and effective use of C.aromaticus leaves for urolithiasis treatment.
References
Atmani F, Slimani Y, Mimouni M, Hacht
B. 2003. Prophylaxis of calcium oxalate
stones by Herniaria hirsute on
experimentally induced nephrolithiasis in
rats. BJU Int 92:137-140.
Fraga CG, Leibovitz BE, Tappel AL. 1988.
Lipid peroxidation measured as
Thiobarbituric acid-reactive substances in
tissue slices: characterization and
comparison with homogenates and
microsomes. Free Radic Biol Med 4: 155-
French JW, Yamanaka BS, Ostwald R.
Dietary induced glomerulosclerosis in
the guinea pig. Arch Pathol 83: 204-220.
Keane WF. 1994. Lipids and the Kidney.
Kidney Int 46: 910-920.
Kikuzaki H, Nakatani N. 1993. Antioxidant
effects of some ginger constituents. J Food
Sci 58: 1407–1410.
Kubo K, Saito M, Tadocoro T, Maekawa A.
Changes in susceptibility of tissues to
lipid peroxidation after ingestion of various
levels of docosahexanoic acid and vitamin
E. Br J Nutr 78: 655–669.
Kumar A. 2007. Mast cell stabilization of
coleus aromaticus leaf extract in rat
peritoneal mast cells. Indian J Pharmacol,
: 119-120.
Menon M, Parulkar BG, Drach GW. 1998.
Urinary lithiasis: etiology, diagnosis, and
medical treatment.(7th ed.). In: Campbell’s
Urology Walsh P C, Retik AB, Vaughan
ED, Wein AJ (eds), vol.3 W.B. Saunders
Co: Philadelphia; 661-733.
Miller NJ, Sampson J, Candeias LP,
Bramley PM, Rice-Evans CA. 1996.
Antioxidant activities of carotenes and
xanthophylls. FEBS Lett 384: 240–242.
Mitra SK, Gopumadhavan S,
Venkataranganna MV, Sandarac R. 1998.
Effect of Cystone, a herbal formulation, on
glycolic acid-induced Urolithiasis.
Phytother Res 12: 372–374.
Moorhead JF, Chan MK, Elnhas M,
Varghese Z. 1982. Lipid nephroptoxicity in
chronic progressive glomerular and tubule
interstitial disease. Lancet 2: 1309-1311.
Moro FD, Mancini M, Tavolini IM, Marco
VD, Bassi P. 2005. Cellular and molecular
gateways to urolithiasis: a new insight. Urol
Int 74: 193–197.
Pak CYC, 1989. Role of medical
prevention. J Urol 141: 798–801.
Pilar P, Manuel P, Miguel A. 1999.
Spectrophotometric Quantitation of antioxidant capacity through the formation of a
Phosphomolybdenum complex: Specific
Application to the Determination of
Vitamin E. Anal Biochem 269: 337-341.
Prasad KVSRG, Bharathi K, Srinivasan KK
Evaluation of Musa (parasidica Linn
Cultivar)-“Puttubale” stem juice for
antilithiatic activity in albino rats. Indian J
Physiol and Pharmacol 37: 337–341.
Prudent D, Perneau F, Bessiere JM.,
Michel. 1995. Analysis of the essential oil
of wild oregano from Martinique (coleus
aromaticus Benth) Evaluation of its bacterio
static and fungi static properties. J essent
Oil Res 7: 165-173.
Satoshi H, Hiroomi S, Tomioka T. 2005.
Kidney stone disease and risk factors for
coronary heart disease. Inl J Urol, 12: 859–
Scheuer H, Gwinner W, Hohbach J, Grone
E F, Brandes R. P, Malle E. 2000. Oxidant
stress in hyperlipidemia-induced renal
damage. Am J Physiol, 278: F63–F74.
Sumathi R, Jayanthi S, Kalpanadevi V,
Varalakshmi P. 1993. Effect of DL alphalipoic acid on tissue lipid peroxidation and
antioxidant systems in normal and
glycollate treated rats. Pharmacol Res, 27:
–318.
Sumathi R, Jayathi S, Varalakshmi P. 1995.
Impaired lipid metabolism in calcium
oxalate stone forming rats and DL-alphaLipoic acid supplementation. Nutr res, 15:
-70.
Varalakshmi G, Shanmugasundaram K R,
Venugopal A. 1977. Blood lipids in
renalstone disorders. Indian J Med Res, 66:
-846.
Vermeulen CW. 1962. Essays in
Experimental Biology. University of
Chicago Press:Chicago; 253–269
Volkan T, Emin O, Bekir A, Serdar A,
Turhan C, Ali I. T. 2007. Manganese
superoxide dismutase (Mn-SOD) gene
polymorphisms in Urolithiasis, Urol Res,
: 219–224.
Vincenzo F, Veronica V, Giacomino R.,
Alberto R. (1999). Method for Measuring
Antioxidant Activity and Its Application to
Monitoring the Antioxidant Capacity of
Wines. J Agric Food Chem, 47: 1035–