Antioxidative and antidiabetic effects of naringin and curcumin in vitro and in vivo
Keywords:
diabetes, naringin, curcumin, antioxidant activity, DNA damageAbstract
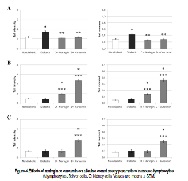
The aim of the present study was to assess whether naringin or curcumin can influence oxidative stress induced DNA damage in mice with alloxan-induced diabetes. Naringin or curcumin preparations (50 mg kg-1) were given intraperitoneally for 7 days. The antioxidant capacity of curcumin and naringin in vitro was evaluated using three assays which cover different aspects of antioxidant activity. In order to evaluate the effectiveness of naringin and curcumin in vivo we observed changes in body weight and survival of diabetic mice and used the comet and micronucleus assays. In vitro curcumin showed appreciable antioxidant properties, while naringin was much less effective. Naringin or curcumin administration to diabetic mice resulted in decreased DNA damage in lymphocytes and increased level of DNA damage in liver, kidney and reticulocytes. Administration of naringin and curcumin resulted in significant increase of the body weight and 100% survival of mice. Results suggests that antioxidant activity of naringin and curcumin leads to long time survival of diabetic mice and possible prevention of further oxidative damage, so they could be candidates for antidiabetic agent, but the precise targets of naringin and curcumin in diabetic mice are still to be clarified.
References
Atkins RC, Zimmet P. Diabetic kidney disease: Act now or pay later. Med J Aust. 2010;192:272-74.
Yue KK, Chung WS, Leung AW, Cheng CH. Redox changes precede the occurrence of oxidative stress in eyes and aorta, but not in kidneys of diabetic rats. Life Sci. 2003;73:2557-70.
Oršolić N, Bašić I. Honey Bee Products and their Polyphenolic Compounds in Tretment of Diabetes. In: Govil JN, Singh VK, editors. Recent Progres in Medical Plants 22, Phytopharmacology and Therapetutic Values IV. USA: Studium Press, LLC; 2008. p. 455-71.
Zhang Y, Zhou J, Wang T, Cai L. High level glucose increases mutagenesis in human lymphoblastoid cells. Int J Biol Sci. 2007;3:375–9.
Oršolić N, Gajski G, Garaj-Vrhovac V, Đikić D, Prskalo ZŠ, Sirovina D. DNA-protective effects of quercetin or naringenin in alloxan-induced diabetic mice. Eur J Pharmacol. 2011;656:110-8.
Oršolić N, Sirovina D, Gajski G, Garaj-Vrhovac V, Jazvinšćak Jembrek M, Kosalec I. Assessment of DNA damage and lipid peroxidation in diabetic mice: Effects of propolis and epigallocatechin gallate (EGCG). Mutat Res-Gen Tox En. 2013;757:36–44.
Oršolić N, Car N. Quercetin and hyperthermia modulate cisplatin-induced DNA damage in tumor and normal tissues in vivo. Tumour Biol. 2014;35(7):6445-54.
Oršolić N, Sirovina D, Zovko Končić M, Lacković G, Gregorović G. Effect of Croatian propolis on diabetic nephropathy and liver toxicity in mice. B.M.C. Complem Altern M. 2012;12:117.
Mokini Z, Marcovecchio ML, Chiarelli F. Molecular pathology of oxidative stress in diabetic angiopathy: Role of mitochondrial and cellular pathways. Diabetes Res Clin Pr. 2010;87:313–21.
Singh U, Jialal I. Oxidative stress and atherosclerosis. Pathophysiology, 13, 129–42.
Tabart J, Kevers C, Pincemail J, Defraigne JO, Dommes J. 2009. Comparative antioxidant capacities of phenolic compounds measured by various tests. Food Chem. 2006;113:1226–33.
Lukačínová A, Mojžiš J, Beňačka R, Keller J, Maguth T, Kurila P, Vaško L, Rácz O, Ništiar F. Preventive Effects of Flavonoids on Alloxan-Induced Diabetes Mellitus in Rats. Acta Vet Brno. 2008;77:175-82.
Jacques PF, Cassidy A, Rogers G, Peterson JJ, Meigs JB, Dwyer JT. Higher Dietary Flavonol Intake Is Associated with Lower Incidence of Type 2 Diabetes. J Nutr. 2013;143:1474-80.
Sirovina D, Oršolić N, Zovko Končić M, Kovačević G, Benković V, Gregorović G. Quercetin vs chrysin: effect on liver histopathology in diabetic mice. Hum Exp Toxicol. 2013;32:1058-66.
Denys JC. Antioxidant Properties of Spices, Herbs and Other Sources. Springer, New York. 2013.
Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Hawkins Byrne D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Comp Anal. 2006;19:669–75.
Oszmianski J, Wojdylo A. Effects of various clarification treatments on phenolic compounds and color of apple juice. Eur Food Res Technol. 2007;224:755–62.
Suárez-Jacobo A, Rüfer CE, Gervilla R, Guamis B, Roig-Sagués AX, Saldo J. Influence of ultra-high pressure homogenisation on antioxidant capacity, polyphenol and vitamin content of clear apple juice. Food Chem. 2011;127:447–54.
Kremer D, Kosalec I, Locatelli M, Epifano F, Genovese S, Carlucci G, Zovko Končić M. Anthraquinone profiles, antioxidant and antimicrobial properties of Frangula rupestris (Scop.) Schur and Frangula alnus Mill. bark. Food Chem. 2012;131:1174–80.
Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175:184–91.
Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–65.
Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–48.
Singh N, Rajini PS. Free radical scavenging activity of an aqueous extract of potato peel. Food Chem. 2004;85:611–6.
Leopoldini M, Russo N, Chiodo S, Toscano M. Iron chelation by the powerful antioxidant flavonoid quercetin. J Agric Food Chem. 2006;54:6343–51.
Yanagisawa D, Shirai N, Amatsubo T, Taguchi H, Hirao K, Urushitani M, Morikawa S, Inubushi T, Kato M, Kato F, Morino K, Kimura H, Nakano I, Yoshida C, Okada T, Sano M, Wada Y, Wada KN, Yamamoto A, Tooyama I. Relationship between the tautomeric structures of curcumin derivatives and their Abeta-binding activities in the context of therapies for Alzheimer's disease. Biomaterials. 2010;31:4179–85.
Chattopadhyay D, Somaiah A, Raghunathan D, Thirumurugan K. Dichotomous Effect of Caffeine, Curcumin, and Naringenin on Genomic DNA of Normal and Diabetic Subjects. Scientifica, vol. 2014, Article ID 649261, 7 pages, doi:10.1155/2014/649261
Martín MÁ, Serrano AB, Ramos S, Pulido MI, Bravo L, Goya L. Cocoa flavonoids up-regulate antioxidant enzyme activity via the ERK1/2 pathway to protect against oxidative stress-induced apoptosis in HepG2 cells. J Nutr Biochem. 2010;21:196–205.
Waisundara VY, Siu SY, Hsu A, Huang D, Tan BKH. Baicalin upregulates the genetic expression of antioxidant enzymes in Type-2 diabetic Goto-Kakizaki rats. Life Sci. 2011;88:1016–25.