Mutagenicity, antimutagenicity and tyrosinase inhibition activity of hydroglycol extracts from Terminalia chebula Retzius, Terminalia bellerica Roxb and Rafflesia kerrii Meijer
Keywords:
Terminalia chebula Retzius, Terminalia bellerica Roxb, Rafflesia kerrii Meijer, genotoxicity, tyrosinase inhibition activityAbstract
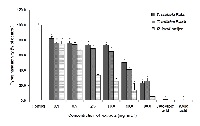
The hydroglycolic extracts from Terminalia chebula Retzius, Terminalia bellerica Roxb and Rafflesia kerrii Meijer were investigated for total phenolic content (TPC), cytotoxicity, mutagenicity, antimutagenicity and antityrosinase for safety assessment as novel botanical-based cosmeceutical ingredients. These plant extracts showed TPC between 14.90 ± 0.02 and 112.40 ± 0.08 mg GAE g-1 of extract when using the Folin-Ciocalteu method. The cytotoxicity study revealed that the 50% cytotoxicity dose (CD50) towards normal mouse fibroblast L929 and mouse melanoma B16F10 cell lines was 5.43 ± 0.18 - 39.39 ± 0.14 mg mL-1 and 4.35 ± 0.33 - 58.23 ± 0.18 mg mL-1, respectively. In genotoxicity investigation, it was found that all extracts were not mutagenic at the concentrations up to 87.34 mg 0.1 mL-1 when tested with Salmonella typhimurium strains TA98 and TA100 in the presence and absence of metabolic activation (S9 microsomal fraction). The extracts were further tested for antimutagenic activity against 2-aminoanthracene (2-AA) and 2-(2-furyl)-3-(5-nitro-2-furyl) acrylamide (AF-2) which were used as the tested mutagens. Interestingly, all hydroglycolic extracts exhibited the inhibitory effect on the mutagenicity after being induced by 2AA and AF-2 in S. typhimurium strains TA98 and TA100 in the presence and absence of metabolic activation. All plant extracts were further investigated for tyrosinase inhibitory activity. Results showed that all extracts possessed tyrosinase inhibitory activity with 50% inhibitory concentration values (IC50) of 1.27 ± 0.49 - 39.96 ± 0.21 mg mL-1. Overall studies including their antimutagenicity and antityrosinase activities suggest that the hydroglycolic extracts of these three plants may be used as potential candidates for skin-care cosmeceutical ingredients.
References
Afaq F, Mukhtar H. Photochemoprevention by botanical antioxidants. Skin Pharmacol Appl Skin Physiol. 2002; 15:297-306.
Svobodova A, Psotova J, Walterova D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003; 147:137-145.
Matsumura Y, Ananthaswamy HN. Toxic effects of ultraviolet radiation on the skin. Toxicol Appl Pharmacol.2004; 195:298-308.
Barthakur NN, Arnold NP. Nutritive value of the chebulic myrobalan (Terminalia chebula Retz.) and its potential as a food source. Food Chem. 1991;40:213-219.
Chattopadhyay RR, Bhattacharyya SK. Terminalia chebula: An update. Pharmacogn Rev. 2007;1:151-156.
Shin TY, Jeong HJ, et al. Inhibitory action of water soluble fraction of Terminalia chebula on systemic and local anaphylaxis. J Ethnopharmacol. 2001;74:133-140.
Saleem A, Husheem M, Harkonen P, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula Retz. fruit. J Ethnopharmacol 2002;81:327-336.
Reddy DB, Reddy TC, et al. Chebulagic acid, a COX-LOX dual inhibitor isolated from the fruits of Terminalia chebula Retz., induces apoptosis in COLO-205 cell line. J Ethnopharmacol.2009;124:506-512.
Kim SJ, Sancheti SA, et al. Effect of 1,2,3,4,6-penta-O-galloyl-beta-D-glucose on elastase and hyaluronidase activities and its type II collagen expression. Acta Pol Pharm. 2010;67:145-150.
Elizabeth KM. Antimicrobial activity of Terminalia bellerica. Indian J Clin Biochem.2005;20:150-153.
Sabu MC, Kuttan R. Antidiabetic and antioxidant activity of Terminalia belerica Roxb. Indian J Exp Biol. 2009;47:270-275.
Kaur S, Michael H, Arora S, Harkonen PL, Kumar S. The in vitro cytotoxic and apoptotic activity of Triphala an Indian herbal drug. J Ethnopharmacol. 2005;97:15-20.
Valsaraj R, Pushpangadan P, et al. New anti-HIV-1, antimalarial, and antifungal compounds from Terminalia bellerica. J Nat Prod. 1997;60:739-742.
Pfundstein B, El Desouky SK, Hull WE, Haubner R, Erben G, Owen RW. Polyphenolic compounds in the fruits of Egyptian medicinal plants (Terminalia bellerica, Terminalia chebula and Terminalia horrida): characterization, quantitation and determination of antioxidant capacities. Phytochem. 2009;71:1132-1148.
Kanchanapoom T, Kamel MS, Picheansoonthon C, Luecha P, Kasai R,Yamasaki K. Hydrolyzable tannins and phenylpropanoid from Rafflesia kerrii Meijer (Rafflesiaceae). J Nat Med. 2007;61:478-479.
Chulasiri M, Wanaswas P, et al. Utilizing hydroglycolic extract from myrobalan fruits to counteract reactive oxygen species. Int J Cosmet Sci. 2011;33:371-376.
Chan EWC, Lim YY, et al. Antioxidant tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008;109:477-483.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55-63.
Maron DM, Ames BN. Revised methods for the Salmonella mutagenicity test. Mutat Res. 1983;113:173-215.
Mortelmans K, Zeiger E. The Ames Salmonella/microsome mutagenicity assay. Mutat Res. 2000;455:29-60.
Kulwat C, Lertprasertsuke N, Leechanachai P, Kongtawelert P, initketkumnuen U. Antimutagenicity and DT-diaphorase inducing activity of Gynostemma pentaphyllum Makino extract. J Med Invest. 2005;52:145-150.
Organization for Economic Co-operation and Development (OECD). Bacterial reverse mutation test. Test No. 471. In: OECD guidelines for testing of chemicals. OECD, Paris (1997).
Baumann L. Botanical ingredients in cosmeceuticals. J Drugs Dermatol. 2007;6:1084-1088.
Lee KT, Kim BJ, Kim JH, Heo MY, Kim HP. Biological screening of 100 plant extracts for cosmetic use (I): inhibitory activities of tyrosinase and DOPA auto-oxidation. Int J Cosmet Sci. 1997;19:291-298.
Kim BJ, Kim JH, Kim HP, Heo MY. Biological screening of 100 plant extracts for cosmetic use (II): anti-oxidative activity and free radical scavenging activity. Int J Cosmet Sci. 1997;19:299-307.
Burapadaja S., Bunchoo A. Antimicrobial activity of tannins from Terminalia citrina. Planta Med. 1995;61:365-366.
Kaur S, Grover IS, Singh M. Antimutagenicity of hydrolyzable tannins from Terminalia chebula in Salmonella typhimurium. Mutat Res. 1998;419:169-179.
Pinmai K, Chunlaratthanabhorn S, Ngamkitidechakul C, Soonthornchareon N, Hahnvajanawong C. Synergistic growth inhibitory effects of Phyllanthus emblica and Terminalia bellerica extracts with conventional cytotoxic agents: doxorubicin and cisplatin against human hepatocellular carcinoma and lung cancer cells. World J Gastroenterol. 2008;14:1491-1497.
Hodek P, Trefil P, Stiborova M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem Biol Interact. 2002;139:1-21.
Webster RP, Gawde MD, Bhattacharya RK. Protective effect of rutin, a flavonol glycoside, on the carcinogen-induced DNA damage and repair enzymes in rats. Cancer Lett. 1996;109:185-191.
Carino-Cortes R, Hernandez-Ceruelos A, Torres-Valencia JM, Gonzalez-Avila M, Arriaga-Alba, M. and Madrigal-Bujaidar, E. Antimutagenicity of Stevia pilosa and Stevia eupatoria evaluated with the Ames test. Toxicol In Vitro. 2007;21:691-697.
Madani A, Jain SK. Anti-Salmonella activity of Terminalia belerica: in vitro and in vivo studies. Indian J Exp Biol. 2008;46:817-821.
del Marmol V, Beermann F. Tyrosinase and related proteins in mammalian pigmentation. FEBS Lett. 1996;381:165-168.
Chang TS. An updated review of tyrosinase inhibitors. Int J Mol Sci. 2009;10:2440-2475.
Kim M, Park J, Song K, Kim HG, Koh J-S, Boo YC. Screening of plant extracts for human tyrosinase inhibiting effects. Int J Cosmet Sci. 2012;34:202-208.