Medicinal plants used for treatment of wounds and skin infections: assessment of wound healing and antimicrobial properties of Mallotus oppositifolius and Momordica charantia
Keywords:
Antimicrobial, antioxidant, antioxidantMomordica charantia, wound healingAbstract
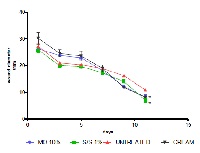
Mallotus oppositifolius and Momordica. charantia are two medicinal plants used in folk medicine for the treatment of skin infections and disorders, wounds, peptic ulcer, fever, piles and parasitic infections. The aim of the study was to determine the antimicrobial, antioxidant and wound healing properties of the methanol leaf extracts of M. oppositifolius and M. charantia. The two plants extracts were screened for their phytochemical composition and their antimicrobial activity was also determined against three gram-positive, two gram-negative bacteria and a fungus. The antioxidant or free radical scavenging activity of the methanol leaf extracts was also determined using 2,2-diphenyl-1-picrylhydrazyl (DPPH). The wound healing activity of the extracts was determined by the excision wound model in rats. The results indicated that both extracts contain tannins, alkaloids, flavonoids and glycosides and M. charantia contains saponins. The two extracts exhibited activity against all the test organisms. MICs of the methanol leaf extract of M. oppositifolius was between 0.01 to 3.75 mg/mL and that of M. charantia was between 1.88 to 7.5 mg/mL. The IC50 of methanol leaf extracts of M. oppositifolius and M. charantia were 16.11 and 7.09 µg/mL respectively. The wound contraction rate was significant (p<0.05) for both extracts of M. oppositifolius and M. charantia on the 11th day compared to the untreated group. The extracts exhibited high antimicrobial and antioxidant activities and increased fibrosis and collagenation in the wound tissues and the rate of wound closure compared with the untreated wounds at the late phase of the wound healing process.
References
Bennett RG. Microbiological considerations of cutaneous surgery. In: Fundamentals of cutaneous surgery, Bennett RG (Editor), C.V. Mosby Company, St. Louis, 1988; p 136.
Myers KA, Marshsl RD, Friedin J. Principles of Pathology in Surgery, 1st ed., Blackwell Scientific Publications: London, 1980; pp 58-62.
Menke WB, Ward KR, Witten TM, Bonchev DG, Diegelmann RF. Impaired wound healing. Clin Dermatol. 2007; 25: 19-25.
Baynes JW. Role of oxidative stress in the development of complications in diabetes. Diabetes. 1991; 40 (4): 405-412.
Jacob RA. The integrated antioxidant system. Nutr Res. 1995; 15:755.
Agyare C, Asase A, Lechtenberg M, Niehues M, Deters A, Hensel A. Ethnopharmacological survey and in vitro confirmation of ethnopharmacological use of medicinal plants used for wound healing. J Ethnopharmacol. 2009; 125, 393-403.
Agyare C, Bempah BS, Boakye YD, Ayande GP, Adarkwa-Yiadom M, Mensah KB. 2013. Evaluation of antimicrobial and wound healing potential of Justicia flava and Lannea welwitschii. Evidence-Based Complement Althern Med. 2013; Article ID 632927, 10 pages.
Agyare C, Lechtenberg M, Deters A, Petereit F, Hensel A. Ellagitannins from Phyllanthus muellerianus (Kuntze) Exell.: geraniin and furosin stimulate cellular activity, differentiation and collagen synthesis of human skin keratinocytes and dermal fibroblasts. Phytomedicine 2011; 18(7): 617-624.
Chukwujekwu JC, van Staden J, Smith P. Antibacterial, anti-inflammatory and antimalarial activities of some Nigerian medicinal plants. South Afri J Bot. 2005; 71 (3-4): 316-325.
Burkill HM. The useful plants of West Tropical Africa. 2nd ed., Families E-I. Royal Botanic Gardens, Kew, Richmond, United Kingdom, vol 2, 1994; p 636.
Atindehou KK, Kone M, Terreaux C, Traore D, Hostettmann K, Dosso M. Evaluation of the antimicrobial potential of medicinal plants from Ivory Coast. Phytotherapy Res. 2002; 16(5): 497-502.
Nwaehujor CO, Ezeja MI, Udeh NE, Okoye DN, Udegbunam RI. Anti-inflammatory and anti-oxidant activities of Mallotus oppositifolius (Geisel) methanol leaf extracts, Arabian J Chem. 2012, http://dx.doi.org/10.1016/j.arabjc.2012.03.014.
Kukuia KE, Ameyaw EO, Mante PK, Adongo DW, Woode E. Screening of central effects of the leaves of Mallotus oppositifolius (Geiseler) Mull. Arg. in mice. Pharmacologia 2012; 3: 683-692.
Braca A, Siciliano T. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoterapia 2008; 79(2):123–125.
Raman A, Lau C. Anti-diabetic properties and phytochemistry of Momordica charantia L. (Cucurbitaceae). Phytomedicine 1996; 2(4): 349-362.
Evans WC. Trease and Evans Pharmacognosy, 16th ed., Elsevier Limited, China. 2009; pp 225- 356.
Wiegand I, Hilpert K, Hancock REW. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nature Protocols 2008; 3, 163-175.
Agyare C, Kuffour GA, Boamah VE, Adu F, Mensah BK, Adu-Amoah L. Antimicrobial and anti-inflammatory activities of Pterygota macrocarpa and Cola gigantia (Sterculiaceae). Evidence-Based Complement Altern Med. 2012; Article ID 902394, 9 pages.
Eloff JN. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998; 64 (8): 711–713.
Chizzola R, Michitsch H, Franz C. Antioxidant properties of Thymus vulgares leaves: Comparison of different extracts and essential oil chemotypes. J Agric Food Chem. 2008; 27, 6897-6904.
Varshney AC, Sharma DN, Mohinder S, Sharma SK, Nigam JM. Therapeutic value of Bovine Saliva in wound healing and histo-morphological study. Indian J Exp Biol. 1997; 35: 535-537.
Thomas DW, Harding KG. Wound healing. Bri J Surg. 2001; 89 (10): 1203–1205.
Guo S, DiPietro LA. Factors affecting wound healing. J Dent Res. 2010; 89 (3): 219-229.
Brown DJ. Herbal prescriptions for health and healing: your everyday guide to using herbs safely and effectively. 1st ed., Lotus Press, 2000; p 38.
Houghton PJ, Hylands PJ, Mensah AY, Hensel A, Deters AM. In vitro tests and ethnopharmacological investigations: wound healing as an example. J Ethnopharmacol. 2005; 100: 100-107.
Andrews JM. Determination of minimum inhibitory concentrations. J Antimicrob Chemother. 2001; 48 (Suppl. 1): 5-16.
Garg MC, Ojha S, Bansal DD. Antioxidant status of streptozotocin-diabetic rats, Indian J Exp Biol. 1996; 34: 264.
Khatoon M, Islam E, Rahman RAA, Alam AHMK, Khondkar P, Rashid M, Parvin S. Estimation of total phenol and in vitro antioxidant activity of Albizia procera leaves. BMC Res. Notes 2013; 6: 121.