Antioxidant and antihyperglycemic potential of methanolic extract of bark of mimusops elengi l. In mice.
Keywords:
Mimusops elengi, antihyperglycemic, antioxidant, DPPH, diabetic OGTTAbstract
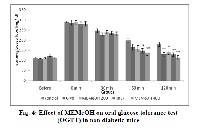
Ayurveda refers Mimusops elengi L. for the treatment of the diabetes. Considering the traditional claim of M. elengi in management of diabetes and the possible involvement of oxidative stress in pathogenesis of diabetes, the present study was aimed to evaluate the in vitro antioxidant and in vivo antihyperglycemic property of methanolic extract of bark of M. elengi (MEMeOH). In vitro antioxidant activity of MEMeOH was evaluated using reducing power assay, DPPH and hydroxyl radical scavenging assay. MEMeOH offered significant in vitro reducing power capacity and radical scavenging activity. In acute study in alloxan induced diabetes, MEMeOH exhibited significant (p< 0.001) antihyperglycemic effect. The onset of antihyperglycemic effect was observed at 2nd hr; peak activity was demonstrated at 6th hr. The antihyperglycemic effect of MEMeOH 400mg/kg, p.o. was persistent up to 24th hr after drug administration. MEMeOH produced significant (p < 0.01) reduction in elevated glucose levels in glucose loaded non diabetic animals. The onset of action in non diabetic oral glucose tolerance test was found to be at 60th min and peak activity was observed at 120th min after oral glucose load. MEMeOH demonstrated significant (p < 0.01) reduction in elevated glucose levels 2hr before glucose administration and 6 hr after glucose load in oral glucose tolerance test in diabetic animals. MEMeOH has demonstrated antihyperglycemic activity in diabetic as well as non diabetic glucose loaded mice. MEMeOH should be further explored against diabetes and related complications.
References
Paul Zimmet KG, Alberti MM. Nature
; 414:781.
Isomaa B, Almgren P, Tuomi T, Forsen B,
Lahti K, Nissen M, et al. Cardiovascular
morbidity and mortality associated with the
metabolic syndrome. Diabetes Care 2001;
: 683– 9.
Koya D, King GL. Protein kinase C
activation and the development of diabetic
complications. Diabetes 1998; 47, 859–866.
Pitozzi V, Giovannelli L, Bardini G, Rotella
CM, Dolara P. Oxidative DNA damage in
peripheral blood cells in type 2 diabetes
mellitus: higher vulnerabiity of polymorpho
nuclear leukocytes. Mutat. Res. 2003; 529,
–133.
Bailey CJ, Day C. Traditional treatments for
diabetes. Diabetes. Care 1989; 12:553–564.
Valiathan MS. Healing plants. Curr. Sci.
; 75:1122–1126.
The WHO Expert Committee on Diabetes
Mellitus, Technical Repot Series World
Health Organization. Geneva;1980.
Arulmozhi DK, Veeranjaneyulu A,
Bodhankar S.L. Neonatal streptozotocin
induced rat model of Type 2 diabetes
mellitus: a glance. Indian J. Pharmacol.
; 36:217–221.
Database on Medicinal Plants used in
Ayurveda, Central Council for Research in
Ayurveda and Siddha, Department of ISM
& H, Ministry of Health and Family
Welfare (Govt. of India) 2000; 65-68.
Chopra RN, Nayar S L and Chopra IC.
Glossary of Indian Medicinal Plants,
National Institute of Science
Communication and Information Resources
(CSIR), New Delhi, 2000; 167.
Joshi SG, Medicinal Plants, Oxford & IBH
publishing Co. Pvt. Ltd. 2000; 362.
Anonymous: The Wealth of India.
Publications and information Directorate,
CSIR, New Delhi, India 1969;03
Sushrut Samhita, Chikitsa-Sthana Chapter
, Prameh Chikitsa, Shlok-10, pg- 447,
Dravya Gun Vidnyan, part-2, Prof. P. V.
Sharma, Plant no. 133, pg- 329- 331.
Velavan S, Nagulendran K, Mahesh R,
Hazeena Begum V. In vitro antioxidant
activity of Asperagus racemosus root.
Pharmacog mag 2002; 26-33.
Segundo MA, Magalhaes L M, Reis S.
Methodological aspects about in vitro
evaluation of antioxidant properties
Analytica Chemical Acta 2008; 613: 1-19.
Latha M, Pari L. Antihyperglycaemic effect
of Cassia auriculata in experimental
diabetes and its effects on key metabolic
enzymes involved in carbohydrate
metabolism. Clin Exp Pharmacol Physiol
; 30: 38-43.
Block, G, Patterson B, Subar A. Fruits,
vegetables, and cancer prevention: a review
of the epidemiological evidence. Nut.
Cancer 1992;18: 1–29.
Houstis N, Rosen ED, Lander ES. Reactive
oxygen species play a causal role in
multiple forms of insulin resistance. Nature
; 440:944–948.
Rahimi R, Shekoufeh N, Bagher L,
Mohammad AA. Review on the role of
antioxidants in the management of diabetes
and its complications.Biomed.
Pharmacother 2005; 59: 365–373.
Exarchou V, Nenadis N, Tsimidou G,
Gerothanassis I P, Troganis A, & Boskou
D.Antioxidant activities and phenolic
composition of extracts from Greek
oregano, Greek sage and summer savory. J
Agri & Food Chem 2002,; 50(19): 5294–
Loliger J .The use of antioxidants in food.
In O. I. Aruoma, & B. Halliwell (Eds.), Free
radicals and food additives 1991; 129–150
Velioglu Y S, Mazza G, Gao L,& Oomah B
D : Antioxidant activity and total phenolics
in selected fruits, vegetables, and grain
products. J Agri Food & Chemistry 1998;
: 4113–4117.
Pietta P G : Flavonoids in medicinal plants.
In C. A. Rice-Evans, & L. Packer (Eds.),
Flavonoids in health and disease, New
York: Dekker 1998; 61–110
Osawa T : Novel natural antioxidants for
utilization in food and biological systems.
In I. Uritani, V. V. Garcia, & E. M.
Mendoza (Eds.), Postharvest biochemistry
of plant food-materials in the tropics.
Tokyo, Japan: Japan Scientific Societies
Press 1994; 241–251.
Hseu YC, Chang WH, Chen CS, Liao JW,
Huang CJ, Lu FJ, Chia YC, Hsu HK, Wu
JJ, Yang HL: Antioxidant activities of
Toona Sinensis leaves extracts using
different antioxidant models. Food and
Chemical Toxicology 2008; 46: 105–114.
Hipeli S and Elstner EF:OH-radical type
reactive oxygen species: a short review on
the mechanisms of OH-radical and
peroxynitrite toxicity. Z. Naturforsch 1997;
C: 555-563
Halliwell B and Gutteridge JM C:
Biologically relevant metal ion-dependent
hydroxyl radical generation. An update.
FEBS Lett 1992; 307: 108-112.
Saravanan R, Pari L. Antihyperlipidemic
and antiperoxidative effect of diasulin, a
polyherbal formulation in alloxan induced
hyperglycemic rats. BMC Complement
Altern Med 2005; 5:1-10.