Effect of Aqueous Extract from The Root Cortex of Aralia elata on Intestinal alpha-Glucosidases and Postprandial Glycemic Response in Mice
Keywords:
Aralia elata, alpha-glucosidase, glycemic response, intestine, root cortex, water extractAbstract
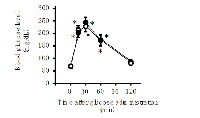
The bark and root cortex of Aralia elata have been used as traditional medicine for the treatment of diabetes mellitus in Oriental countries including China, Korea and Japan. The purpose of this study was to assess the influence of aqueous extract from the root cortex of Aralia elata on the intestinal alpha-glucosidase activity and the glycemic response after ingestion of carbohydrates. In in vitro intestinal alpha-glucosidase assay, water extract from the root cortex of Aralia elata inhibited the activities of intestinal maltase and trehalase in a concentration-dependent manner with IC50 values of 0.45 mg/mL and 0.65 mg/mL, respectively. The fraction which was extracted with water from the root cortex of Aralia elata has a stronger inhibitory effect on the intestinal alpha-glucosidases than that extracted with pure ethanol. In in vivo postprandial glycemic response study using mice, the water extract from the root cortex of Aralia elata (0.125 g/kg) significantly inhibited the increase in plasma glucose concentration induced by oral administration of maltose and trehalose, but not glucose. The observation indicates that the reduced glucose response to disaccharides in mice with the water extract was, at least in part, due to the inhibition of intestinal alpha-glucosidase activity. These findings might support the usefulness of a water-based preparation of the root cortex of Aralia elata, which has been long used in traditional medicine to treat diabetes mellitus.
References
Saito S, Sumita S, Tamura N, Nagamura Y, Nishida K, Ito M, Ishiguro I. Saponins from the leaves of Aralia elata SEEM. (Araliaceae). Chem Pharm Bull. 1990; 38(2): 411-414.
Yoshikawa M, Harada E, Matsuda H, Murakami T, Yamahara J, Murakami N. Elatosides A and B, potent inhibitors of ethanol absorption in rats from the bark of Aralia elata Seem: the structure-activity relationships of oleanolic acid oligoglycosides. Chem Pharm Bull. 1993; 41(11): 2069-2071.
Chung YS, Choi YH, Lee SJ, Choi SA, Lee JH, Kim H, Hong EK. Water extract of Aralia elata prevents cataractogenesis in vitro and in vivo. J. Ethnopharmacol. 2005; 101: 49-54.
Lee EB, Kim OJ, Kang SS, Jeong C. Araloside A, an antiulcer constituent from the root bark of Aralia elata. Biol Pharm Bull. 2005; 28(3): 523-526.
Mizuno T, Tanaka T. Aralia elata. Nippon-Yakuso-Zensho (Encyclopedia of Japanese Medicinal Plants). Nagoya, Japan: Shin-Nippon-Hoki Publishing Co., LTD.; 1995. p. 382–384.
Yoshikawa M, Yoshizumi S, Ueno T, Matsuda H, Murakami T, Yamahara J, Murakami N. Medicinal foodstuffs. I. Hypoglycemic constituents from a garnish foodstuff "taranome," the young shoot of Aralia elata SEEM.: elatosides G, H, I, J, and K. Chem Pharm Bull. 1995; 43(11): 1878-1882.
Chung C, Jung M. Ethanol fraction of Aralia elata Seemann enhances antioxidant activity and lowers serum lipids in rats when administered with benzo()pyrene. Biol Pharm Bull. 2003; 26(10): 1502-1504.
Kaimoto T, Shibuya M, Maeda H. Effects of dietary sprouts of Aralia elata SEEM. on the serum and liver lipid profiles in rats. Jpn J Nutr Diet., 2010; 68(5): 309-314.
Yoshikawa M, Matsuda H, Harada E, Murakami T, Wariishi N, Yamahara J, Murakami N. Elatoside E, a new hypoglycemic principle from the root cortex of Aralia elata SEEM.: structure-related hypoglycemic activity of oleanolic acid glycosides. Chem Pharm Bull. 1994; 42(6): 1354-1356.
Yoshikawa M, Murakami T, Harada E, Murakami N, Yamahara J, Matsuda H. Bioactive saponins and glycosides. VII. On the hypoglycemic principles from the root cortex of Aralia elata SEEM.: structure related hypoglycemic activity of oleanolic acid oligoglycoside. Chem Pharm Bull. 1996; 44(10): 1923-1927.
Caspary WF, Graf S. Inhibition of human intestinal -glucosidehydrolases by a new complex oligosaccharide. Res Exp Med. 1979; 175: 1-6.
van de Laar FA. Alpha-glucosidase inhibitors in the early treatment of type 2 diabetes. Vasc Health Risk Manag. 2008; 4(6): 1189-1195.
Miyahara C, Miyazawa M, Satoh S, Sakai A, Mizusaki S. Inhibitory effects of mulberry leaf extract on postprandial hyperglycemia in normal rats. J Nutr Sci Vitaminol. 2004; 50: 161-164.
Mai TT, Van Chuyen N. Anti-hyperglycemic activity of an aqueous extract from flower buds of Cleistocalyx operculatus (Roxb.) Merr and Perry. Biosci Biotechnol Biochem. 2007; 71(1): 69-76.
Kato A, Minoshima Y, Yamamoto J, Adachi I, Watson AA, Nash RJ. Protective effects of dietary chamomile tea on diabetic complications. J Agric Food Chem. 2008; 56: 8206-8211.
Iwai K. Antidiabetic and antioxidant effects of polyphenols in brown alga Ecklonia stolonifera in genetically diabetic KK-Ay mice. Plant Foods Hum Nutr. 2008; 63: 163-169.
Gholamhoseinian A, Fallah H, Sharifi far F. Inhibitory effect of methanol extract of Rosa damascena Mill. flowers on -glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine. 2009; 16: 935-941.
Kim SH, Jo SH, Kwon YI, Hwang JK. Effects of onion (Allium cepa L.) extract administration on intestinal α-glucosidases activities and spikes in postprandial blood glucose levels in SD rats model. Int J Mol Sci. 2011; 12: 3757-3769.
Mohamed Sham Shihabudeen H, Hansi Priscilla D, Thirumurugan K. Cinnamon extract inhibits α-glucosidase activity and dampens postprandial glucose excursion in diabetic rats. Nutr Metab. 2011; 8: 46.
Dahlqvist A. Determination of maltase and isomaltase activities with a glucose-oxidase reagent. Biochem J. 1961; 80: 547-551.
Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951; 193(1): 265-275.
Takeuchi M, Takai N, Asano N, Kameda Y, Matsui K. Inhibitory effect of validamine, valienamine and valiolamine on activities of carbohydrases in rat small intestinal brush border membranes. Chem Pharm Bull. 1990; 38(7): 1970-1972.
Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes. 2005; 54: 1-7.
Mukherjee A, Sengupta S. Characterization of nimbidiol as a potent intestinal disaccharidase and glucoamylase inhibitor present in Azadirachta indica (neem) useful for the treatment of diabetes. J Enzyme Inhib Med Chem. 2012; in press.