Anticonvulsive and antioxidant activity of aqueous root extract of Moringa oleifera in ferric chloride-induced epileptic rats
Keywords:
Moringa oleifera, free radical, FeCl3-induced epilepsy, neurotransmitter, brain damage, seizureAbstract
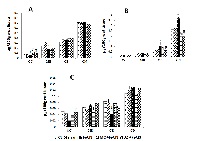
Moringa oleifera (MO), commonly known as drumstick tree in South Asian countries are consumed as food and have immense medicinal value. The consumption of the root of MO reduced neuronal hyper-excitability in psychiatric disorders. The present study has tested the efficacy of the aqueous root extract of MO in preventing epilepsy, a serious neurological disorder, from non-penetrating brain injury. Reducing power, polyphenol, flavonoid content of MO, and high performance liquid chromatographic identification of free radical scavenging compounds in the extract in the present study encourages its use for preventing free radical-induced epilepsy. Holtzman Strain adult rats, weighing 200-250 g, were assigned into four groups (n=6 in each group): normal control; sham operated; intracortically FeCl3 injected (100mM; 8micro litre); FeCl3 injected + MO pretreated (350mg/kg, orally) and FeCl3 injected + diazepam (DZ) pretreated (20mg/kg, i.p). Lipid peroxidation (LPO), catalase (CAT), superoxide dismutase (SOD) activities were studied as indirect parameters of free radical-induced brain damage. Serotonin, dopamine and nor-epinephrine were also evaluated biochemically from different brain regions. A statistically significant reduction in lipid peroxidation, CAT and SOD activity was observed in MO pretreated group when compared to the untreated epileptic rats. Serotonin level was found to be elevated significantly in cerebral cortex whereas dopamine and nor-epinephrine levels declined in the caudate nucleus and in cerebellum of MO-pretreated rats in comparison to untreated epileptic group and synchronizes with the changes with anticonvulsant diazepam. MO effectively prevents the advent of FeCl3 induced epilepsy by ameliorating free radical damage and by regulating protective neurotransmitters to restrain neuronal hyper excitability.
References
. Gupta YK, Gupta M. Post traumatic
epilepsy: A review of scientific
evidence. Indian J Physiol Pharmacol.
; 50: 7-16.
. Willmore LJ, Rubin JJ. Effects of
antiperoxidants on FeCl3-induced lipid
peroxidation and focal edema in rat
brain. Exp Neurol. 1984;83: 62-70.
http://dx.doi.org/10.1016/0014-
(84)90046-3
. Gallagher D. Post-traumatic Epilepsy:
An overview. Einstein Q J Biol Med.
;19: 5-9.
. Scheuer ML, Pedley TA. The
evaluation and treatment of seizures.
N Engl J Med. 1990;323: 1468-1474.
http://www.nejm.org/doi/full/10.1056/N
EJM199011223232107
. Hernandez TD. Preventing posttraumatic epilepsy after brain injury:
weighing the costs and benefits of
anticonvulsant prophylaxies. Trends
Pharmacol Sci. 1997; 18(2): 59-62.
http://dx.doi.org/10.1016/S0165-
(97)89801-X
. Hazra R, Ray K, Guha D. Inhibitory
role of Acorus calamus in ferric
chlorideă induced epileptogenesis in
rat. Hum Exp Toxicol. 2007; 26(12):
-953.
http://het.sagepub.com/content/26/12/
full.pdf+html
. Meurs A, Clinckers R, Ebinger G,
Michotte Y, Smolders I. Seizure
activity and changes in hippocampal
extracellular glutamate, GABA,
dopamine and serotonin. Epilepsy
Res. 2008;78(1): 50-59.
http://www.epiresjournal.com/article/S0920-
%2807%2900320-8/abstract.
. Lancelot E, Lecunu L, Revaud ML,
Boulu RG, Plotkine M, Callebert J.
Glutamate induces hydroxyl radical
formation in vivo via activation of nitric
oxide synthase in Sprague-Dawley
rats. Neurosci Lett. 1998;242(3): 131-
http://www.sciencedirect.com/science/
article/pii/S0304394098000950
. Tripathi PP, Di Giovannantonio LG,
Viegi A, Wurst W, Simeone A, Bozzi
Y. Serotonin hyperinnervation
abolishes seizure susceptibility in otx2
conditional mutant mice. J Neurosci.
;28(37): 9271-9276.
http://www.jneurosci.org/content/28/37
/9271.full.pdf+html
. Kabuto H, Yokoi I, Ogawa N.
Melatonin inhibits iron-induced
epileptic discharges in rats by
suppressing peroxidation. Epilepsia.
;39(3): 237-243.
http://onlinelibrary.wiley.com/doi/10.11
/j.1528-1157.1998.tb01367.x/pdf
. Fahey JW. Moringa oleifera: A review
of the medical evidence for its
nutritional, therapeutic and
prophylactic properties. Part1. 2005.
http://www.TFLJournal.org/article.php/
;1:5
. Debnath S, Biswas D, Ray K, Guha D.
Moringa oleifera induced potentiation
of serotonin release by 5-HT3
receptors in experimental ulcer model.
Phytomedicine. 2011; 18: 91-95.
http://dx.doi.org/10.1016/j.phymed.20
06.003
. Ray K, Hazra R, Guha D. Central
inhibitory effect of Moringa oleifera
root extract: possible role of
neurotransmitters. Indian J Exp Biol.
; 41:1279-1284.
. Ray K, Guha D. Effect of Moringa
oleifera root extract on penicillininduced epileptic rats. Biogenic
Amines. 2005;19:223-231.
. Bhattacharjee AK, Das AK.
Phytochemical screening of some
Indian plants. Quart J Crude Drug
Res. 1969; 9: 1408-1413.
. Liao JF, Sung YH, Yiing MJ, Lili Y,
Chieh FC. Central inhibitory effects of
water extract of Acori grameneai
rhizome in mice. J Ethnopharmacol.
; 61(3): 185-191.
http://dx.doi.org/10.1016/S0378-
(98)00042-7
. Ray K, Hazra R, Debnath PK, Guha
D. Role of 5-Hydroxytryptamine in
Moringa oleifera induced potentiation
of pentobarbitone hypnosis in albino
rats. Indian J Exp Biol. 2004; 4: 632-
. Oyaizu M. Studies on product of
browning reaction prepared from
glucose amine. Jpn J Nutr. 1986; 44:
-315.
. Peőarrieta JM, Alvarado JA, Åkesson
B, Bergenståhl B. Total antioxidant
capacity and content of flanonoids
and other phenlic compoundsin
canihua (Chenopodium pallidicaule):
An Andean pseuodocereal. Mol Nutr
Food Res. 2008; 52(6): 708-717.
http://onlinelibrary.wiley.com/doi/10.10
/mnfr.200700189/abstract
. Nishimiki M, Ra NA, Yagi K. The
occurrence of superoxide anion in the
reaction of reduced phenazine
methosulphate and molecular oxygen.
Biochem Biophys Res Commun.
; 46(2): 849-853.
http://dx.doi.org/10.1016/S0006-
X(72)80218-3
. Marcocci L, Maguire JJ, Droy-Lefaix
MT, Packer L. The nitric oxide
scavenging property of Ginglo biloba
extract Egb 761. Biochem Biophys
Res Commun. 1994; 201(2): 748-755.
http://dx.doi.org/10.1006/bbrc.1994.17
. Lanthorn T, Isaacson RL. Studies of
kainate-induced wet-dog shakes in
the rat. Life Sci. 1978; 22(2): 171-177.
http://dx.doi.org/10.1016/0024-
(78)90534-9
. Misra HP, Fridorich I. The generation
of radical during superoxide
autooxidation of hemoglobin. J Biol
Chem. 1972; 247(21): 6960-6962.
http://www.jbc.org/content/247/21/696
full.pdf+html
. Cohen G, Dembiec D, Mercus J.
Measurement of catalase activity in
tissue extract. Anal Biochem. 1970;
: 30-38.
http://dx.doi.org/10.1016/0003-
(70)90083-7
. Bhattacharya SK, Bhattacharya A,
Das K, Muruganandam AV, Sairam K.
Further investigatigations on the
antioxidant activity of Ocimum
sanctum using different paradigms of
oxidative stress in rats. J Natural
Remedies. 2001;1: 6-16.
. Lowry OH, Rosebrough NJ, Farr AL,
Randall RJ. Protein measurement
with Folin phenol reagent. J Biol
Chem. 1951; 193(1): 265-275.
http://www.jbc.org/content/193/1/265.f
ull.pdf+html
. Kasolo JN, Bimenya GS, Ojok L,
Ogwal-okeng JW. Phytochemicals
and acute toxicity of Moringa oleifera
roots in mice. J Pharmacognosy
Phytother. 2011; 3(3): 38-42.
http://www.academicjournals.org/JPP/
PDF/Pdf2011/April/Kasolo%20et%20a
l.pdf
. Pal D, Sannigrahi S, Majumder UK.
Analgesic and anticonvulsant effect of
saponin isolated from leaves of
Clerodendrum infortunatum Linn. in
mice. Indian J Exp Biol. 2009; 47(9):
-747.
http://nopr.niscair.res.in/bitstream/123
/5978/1/IJEB%2047%289%29
%20743-747.pdf
. Chen YF, Roan HY, Lii CK, Huang
YC, Wang TS. Relationship between
antioxidant and antiglycation ability of
saponins, polyphenols, and
polysaccharides in Chinese herbal
medicines used to treat diabetes. J
Med Plants Res. 2011; 5(11): 2322-
http://www.academicjournals.org/jmpr/
PDF/pdf2011/4June/Chen%20et%20a
l.pdf
. Mori A, Yokoi I, Noda Y, Willmore LJ.
Natural antioxidants may prevent
posttraumatic epilepsy: A proposed
based on experimental animal
studies. Acta Med Okayama. 2004;
(3): 111-118.
http://ousar.lib.okayamau.ac.jp/amo/vol58/iss3/1
. Willmore LJ, Sypert GW, Munson JB.
Recurrent seizures induced by cortical
iron injection: A model of
posttraumatic epilepsy. Ann Neurol.
; 4(4): 329-336.
http://onlinelibrary.wiley.com/doi/10.10
/ana.410040408/abstract
. Tome AR, Ferreira PMP, Freitas RM.
Inhibitory action of antioxidants
(ascorbic acid or đ-tocopherol) on
seizures and brain damage induced
by pilocarpine in rats. Arq
Neuropsiquiatr. 2010; 68(3): 355-361.
http://www.scielo.br/pdf/anp/v68n3/v6
n3a05.pdf
. Pasini A, Tortorella A, Gale K. The
anticonvulasant action of fluoxetine in
substantia nigra is dependent upon
endogenous serotonin. Brain Res.
; 724(1): 84-88.
http://dx.doi.org/10.1016/0006-
(96)00291-0
. Brenan TJ, Seeley WW, Kilgard M,
Schreiner CE, Tecott LH. Soundinduced seizures in serotonin 5HT2C
receptor mutant mice. Nature
Genetics. 1997; 16(4): 387-390.
http://www.nature.com/ng/journal/v16/
n4/pdf/ng0897-387.pdf
. Bozzi Y, Vallone D, Borrelli E.
Neuroprotective role of dopamine
against hippocampal cell death. J
Neurosci. 2000; 20(22): 8643-8649.
http://www.jneurosci.org/content/20/22
/8643.full.pdf+html
. Starr MS. The role of dopamine in
epilepsy. Synapse. 1996; 22(2): 159-
http://onlinelibrary.wiley.com/doi/10.10
/%28SICI%291098-
%28199602%2922:2%3C159::AI
D-SYN8%3E3.0.CO;2-C/abstract
. Grutta VL, Sabatino M. Substantia
nigra-mediated anticonvulsant action:
a possible role of a dopaminergic
component. Brain Res. 1990; 515(1-
: 87-93.
http://dx.doi.org/10.1016/0006-
(90)90580-5
. Chauvel P, Trottier S. Role of
noradrenergic ascending system in
extinction of epileptic phenomenon.
Adv Neurol. 1986; 44: 475-487.
. Barry DI, Kikvadze I, Brundin P,
Bolwig TG, Björklund A, Lindvall O.
Grafted noradrenergic neurons
suppress seizure development in
kindling-induced epilepsy. Proc Natl
Acad Sci USA. 1987; 84(23): 8712-
http://www.pnas.org/content/84/23/87
full.pdf+html