Free radical scavenging effect of Trichodesma amplexicaule against renal vascular complications in diabetic Rats
Keywords:
Antioxidant, Trichodesma amplexicaule, STZ, Diabetic, NephropathyAbstract
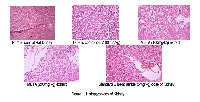
Objective: The aim of the present study was to investigate the antioxidant and renal protective effect of Trichodesma amplexicaule extract in streptozotocin-induced diabetic rats. Methods: Wistar rats were randomly divided into five groups: Normal control, diabetic control and Trichodesma amplexicaule (100 and 200mg/kg) and glibenclamide treated groups. Blood glucose, Total Cholesterol, Triglycerides, Aminotransferases, kidney functions and kidney oxidant/antioxidants status were estimated. Streptozotocin effectively altered the biochemical, histoarchitecture, malondialdehyde and antioxidant enzyme levels, while Trichodesma amplexicaule ameliorated their levels in the treated group. Results: Oral treatment with methanolic extract for 4 weeks reduced significantly Blood glucose, Total Cholesterol, Triglycerides, Aminotransferases, kidney function parameter levels in diabetic animals. Furthermore, the biochemical findings were supplemented with histopathological examination of pancreas and kidney. Histological observation of kidney of diabetic rats showed degenerative changes in glomerulus and renal tubules and extract treatment rejuvenated kidney histoarchitecture. Results of this study suggest that Trichodesma amplexicaule offered significant protection against oxidative stress induced by Streptozotocin, as noted by increase in superoxide dismutase and catalase along with decrease in lipid peroxidation levels. Conclusion: The present work provide scientific evidence of the preventive and therapeutic potential of Trichodesma amplexicaule against kidney disorder in diabetes and thus may provide a promising drug for managing diabetic kidney disorders and assumes that activity may be due to the prominent antioxidant property shown by Trichodesma amplexicaule.
References
. Hovind P, Tarnow L, Rossing
K, Rossing P, Eising S, Larsen N,
Binder C, Parving HH. Decreasing
incidence of severe diabetic
microangiopathy in type 1 diabetes.
Diabetes Care. 2003; 26 (4): 1258ă
. Yokoyama H, Okudaira M, Otani
T, Sato A, Miura J, Takaike H,
Yamada H, Muto K, Uchigata Y,
Ohashi Y, Iwamoto Y. Higher
incidence of diabetic nephropathy in
type 2 than in type 1 diabetes in earlyonset diabetes in Japan. Kidney
International. 2000; 58(1): 302ă311.
. Ramachandran A. Socio-economic
burden of diabetes in India. J Assoc
Physicians India. 2007; 55: 9-12
. Kanwar YS, Wada J, Sun L, Xie P,
Wallner EI, Chen S, Chugh S,
Danesh FR. Diabetic nephropathy:
Mechanisms of renal disease
progression. Exp. Biol. Med. 2008;
: 4ă11.
. Najafian B, Mauer M. Progression of
diabetic nephropathy in type 1
diabetic patients. Diab.Res. Clin.
Pract. 2009; 83: 1ă8.
. Jonasson O, Jones CW, Bauman ABS,
John E, Manaligod J, Tso MOM. The
Pathophysiology of experimental
insulin-deficient diabetes in the
monkey: implications for pancreatic
transplantation. Ann Surg. 1984;
:27-39.
. Sabeeha Shafi, Nahida Tabassum,
Feroz Ahmad. Diabetic Nephropathy
and herbal medicines. International
Journal of Phytopharmacology. 2012;
(1): 10-17.
. Koya D, Hayashi K, Kitada
M, Kashiwagi A, Kikkawa R, Haneda
M. Effects of antioxidants in diabetesinduced oxidative stress in the
glomeruli of diabetic rats. J Am Soc
Nephrol. 2003; 14(8- 3): S250-3.
. Oberley LW. Free radicals and
diabetes. Free Radical Biology and
Medicine. 1988; 5(2): 113ă124.
. Matteucci E and Giampietro O.
Oxidative stress in families of type 1
diabetic patient. Diabetes Care. 2000;
(8): 1182ă1186.
. Baynes JW and Thorpe SR. The role
of oxidative stress in diabetic
complications. Current Opinion in
Endocrinology and Diabetes. 1997;
(4): 277ă284.
. Lipinski B. Pathophysiology of
oxidative stress in diabetes mellitus.
Journal of Diabetes and Its
Complications. 2001; 15(4): 203ă210.
. Kirtikar KR and Basu BD. Indian
Medicinal Plants. Srisatguru
Publications; Delhi; 2000.p. 2335-
. Parrotta AJ. Healing Plants of
Peninsular India. CABI Publishing,
New York; 2001.p.185.
. Chopra RN, Chopra IC, Handa KL
and Kapur LD. Indigenous Drugs of
India. U.N. Dhur and Sons Pvt. Ltd.,
Culcutta; 1958.p.528.
. Varier SPV. Indian Medicinal Plants.
Orient Longman Ltd., Madras;
p.316-317.
. Perianayagam JB, Sharma SK, Pillai
KK. Anti-inflammatory activity of
Trichodesma indicum root extract in
experimental animals. J
Ethnopharmacol. 2006; 104(3): 410-4.
. Perianayagam JB., Sharma SK, Pillai
KK. Antidiarrhoeal evaluation of
Trichodesma indicum root extract.
Methods Finds Exp. Clin. Pharmacol.
; 27(8): 533-537.
. Perianayagam JB, Sharma SK, Pillai
KK. Evaluation of Analgesic and
Antipyretic Potential of Trichodesma
indicum Root Extract in Animal
Models. International Journal of
Pharmaceutical Sciences Letters.
; 1:1-6.
. Khan T, Ahmad MA, Waqar S, Qazi
N. Preliminary evaluation of the
antispasmodic and lipoxygenase
inhibitory effects of some selected
medicinal plants. Pharmaceutical
Biology (Formerly International
Journal of Pharmacognosy). 2009;
(12): 1137- 1141.
. Singh B, Singh S. Antimicrobial
activity of Terpenoids from
Trichodesma amplexicaule Roth.
Phytother Res. 2003;17(7): 814-6.
. Srikanth K, Murgesan T, Kumar Ch
Anil, Suba V, Das AK, Sinha S,
Arunachalam G, Manikandan L. Effect
of Trichodesma indicum extract on
cough reflex induced by sulphur
dioxide in mice. Phytomedicine. 2002;
(1): 75-77.
. Hasan M, Ahamad S, and Mohamood
K. Chemical investigation of
Trichodesma indicum leaves. I.
Nonsteroidol constituents of the
petroleum ether extract. J. Chem.Soc.
; 4(4): 281-283.
. Sudharshan RD, Srinivasa RA and
Pradeep HA. Invitro Antioxidant and
Glucose uptake effect of Trichodesma
indicum in L-6 cell lines. Int J Pharm
Bio Sci. 2012; 3(4): 810 ă 819.
. Ghosh MN. Fundamentals of
experimental pharmacology. Calcutta:
Scientific Book Agency.1984.
. Elizabeth HH. Elevated Liver Function
Tests in Type 2 Diabetes. Clinical
Diabetes. 2005;23(3): 115-19.
. Ohkawa H, Ohishi N, Yagi K. Assay
for lipid peroxides in animal tissue by
Thiobarbituric acid reaction. Anal
Biochem. 1979;95: 351.
. Luck H. Methods of enzymetic
analysis. Academic Press, New York;
p.885.
. Kono Y. Generation of Superoxide
radical during autooxidation of
hydroxylamine and an assay for
superoxide dismutase. Arch Biochem
Biophys.1978;186: 189.
. The Diabetes Control and
Complications Trial Research Group.
The effect of intensive treatment of
diabetes on the development and
progression of long-term
complications in insulin-dependent
diabetes mellitus. New Engl J Med.
;329: 977ă986.
. UK Prospective Diabetes Study
(UKPDS) Group. Intensive bloodglucose control with sulphonylureas or
insulin compared with conventional
treatment and risk of complications in
patients with type 2 diabetes (UKPDS
. Lancet.1998; 352: 837ă853.
. Rösen P, Nawroth PP, King G, Möller
W, Tritschler HJ, Packer L. The role of
oxidative stress in the onset and
progression of diabetes and its
complications: a summary of a
congress series sponsored by
UNESCO-MCBN, the American
Diabetes Association and the German
Diabetes Society. Diabetes Metab
Res Rev. 2001;17: 189ă212.
. Chen V, Ianuzzo CD. Dosage effect
of streptozotocin on rat tissue enzyme
activities and glycogen concentration.
Can J Physiol Pharmacol. 1982;60:
-1256.
. Rajkumar L, Srinivasan N,
Balasubramanian K, Govindarajulu.
Increased degradation of dermal
collagen in diabetic rats. Indian J Exp
Biol. 1991;29:1081-1083.
. Sun L, Halaihel N, Zhang W, Rogers
T, and Levi M. Role of sterol
regulatory element-binding protein 1
in regulation of renal lipid metabolism
and glomerulosclerosis in diabetes
mellitus. J Biol Chem. 2002;
:18919ă18927.
. Rogers K S, Higgins E S & Kline ES.
Experimental diabetes causes
mitochondrial loss and cytoplasmic
enrichment of pyridoxal phosphate
and aspartate aminotransferase
activity. Biochem. Med. Metab. Biol.
;36(1): 91-7.
. Voss C, Brachmann K. & Hartmann
K. Effect of Streptozotocin on
transaminases, creatinine and urea in
serum of rats. Exp. Clin. Endocrinol.
;92(1):37-42.
. JangYY, Song JH, Shin YK, Han ES,
Lee CS. Protective effect of boldine
on oxidative mitochondrial damage in
STZ-induced diabetic rats. Pharmacol
Res. 2000; 42: 361-371.
. Waltner-Law ME, Wang XL, Law BK,
Hall RK, Nawano M et al.
Epigallocatechin gallate, a constituent
of green tea, represses hepatic
glucose production. J Biol Chem.
;277: 34933-34940.
. El-Alfy AT, Ahmed AAE, Fatani AJ.
Protective effect of red grape seeds
proanthocyanidins against induction of
diabetes by alloxan in rats. Pharmacol
Res. 2005;52: 264-270.
. Saravanan R, Pari L.
Antihyperlipidemic and
antiperoxidative effect of Diasulin, a
polyherbal formulation in alloxan
induced hyperglycemic rats. BMC
Complement Altern Med. 2005; 5; 14.