In vitro antisickling activities of two indigenous plant recipes in Ibadan, Nigeria
Keywords:
Sickle cell diseases, indigenous recipes, antisickling activity, Phyllanthus amarus, Harungana madagascariensis, Tetracera potatoria, NigeriaAbstract
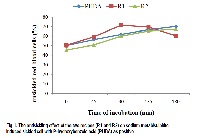
In view of the prevalence sickle cell disease in Nigeria, the use of herbs for treatment of diseases due to high poverty level and inadequate distribution of health care centres in the country, two indigenous recipes consisting of six ethnobotanicals used in the management of sickle cell anaemia in Nigeria were screened for antisickling activity. Recipe 1 constituents were Detarium microcarpum (Guill. and Perr.), Harungana madagascariensis (Lam. ex Poir), Sorghum bicolor (Linn.), Tetracera potatoria (Afzel. ex G. Don) and Theobroma cacao (Linn.). Recipe 2 was a monorecipe of Phyllanthus amarus (Schum and Thonn). Clinical blood samples of confirmed non-crisis sickle cell patients (male and female) were collected from the Department of Haematology, University College Hospital (UCH), Ibadan, Nigeria. Extracts of the recipes were prepared in 80% ethanol using cold extraction method. Sickling of HbSS red blood cells was induced using sodium metabisulphite. Invitro antisickling activities of the crude extracts of the recipes were evaluated using phydroxybenzoic acid and normal saline as positive and negative control respectively. Readings were taken at different incubation time (0 – 180 min). The two recipes showed varied antisickling activities. At 90 min incubation period, Recipe 1 showed maximum percentage inhibition of 71.6 ± 1.21 while Recipe 2 showed 60.0±0.51 percentage inhibition. The inhibitory activity of Recipe 1 at 90 min incubation period was higher than that of the positive control (61.8 ± 2.28) and there was no significant difference (P < 0.5) in the inhibitory activity of Recipe 2 and the positive control. All plant constituents of the two recipes contained saponins and tannins (except Detarium microcarpum). The two indigenous recipes significantly demonstrated antisickling activity. The results of this study validate the use of the two recipes by indigenous people for the treatment of sickle cell disease. The isolation and identification of active phytochemicals responsible for the antisickling activity of Recipe 2 could lead to the discovery of novel drugs
References
. Illustrated Medical Dictionary, 1st ed.
Dorling Kindersley limited, London.
(2002).
. www.nhlbi.nih.gov/health/dci/disease
s/sca/sca. what_is_sickle cell
anaemia?. Retrieved on 30th April,
, 10:20am.
. World Health Organization (WHO),
Sickle Cell Anaemia: Report by the
secretariat. 59th World Health
Assembly. 2006. A59/9.
. Bianchi N, Zuccato C, Lampronti I,
Borgatti and Gambari, R. Fetal
Haemoglobin Inducers from the
Natural World: A novel approach for
identification of Drugs for the
treatment of β-Thalassemia and
Sickle Cell Anaemia, eCAM 2009;
(2):141–151.
. Ballas SK, Marcolina MJ.
Hyperhemolysis during the evolution
of uncomplicated acute painful
episodes in patients with sickle cell
anemia. Transfusion. 2006; 46 (1):
-110.
. Egunyomi A, Moody JO, Eletu OM.
Antisickling activities of two
ethnomedicinal plant Recipes used
for the sickle cell anaemia in Ibadan,
Nigeria. African Journal of
Biotechnology 2009; 8(1): 20-25.
. Wambebe C, Khamofu H, Momoh JA.
Double-blind, placebo-controlled,
randomised cross-over clinical trial
of NIPRISAN in patients with sickle
cell disorder. Phytomedicine. 2001;
(4):252-61.
. Ouattara B, Angenot L, Guissou P,
Fondu P, Dubois J, Frédérich M,
Jansen O, van Heugen J, Wauters
J. Tits M. LC/MS/NMR analysis of
isomeric divanilloylquinic acids from
the root bark of Fagara
zanthoxyloides Lam.
Phytochemistry. 2004; 65(8):1145-
. Mgbemene CN, Ohiri FC. Antisickling potential of Terminalia
catappa leaf extract. Pharmaceutical
biology. 1999; 37(2):152-154.
. Adejumo OE, Adelodun LK.,
Oladimeji PR, Lateef SK. In vitro
antisickling activities and
phytochemical evaluation of
Plumbago zeylanica and Uvaria
chamae. Afr. J. Biotechnol., 2010;
(53): 9032-9036.
. Keay RWJ. Trees of Nigeria.
Revised edn. Clarendon Press,
Oxford. 1989. p. 206.
. Tona L, Kambu K, Ngimbi N, Mesia
K, Penge O, Lusakibanza M,
Cimanga K, De Bruyne T, Apers S,
Totte J, Pieters L, Vlietinck AJ.
Antiamoebic and spasmolytic
activities of extracts from some
antidiarrhoeal traditional
preparations used in Kinshasa,
Congo. Phytomedicine. 2000; 7: 31-
. Erah PO, Asonye CC, Okhamafe
AO. Response of Trypanosoma
brucei brucei– induced anaemia to a
commercial herbal preparation.
African Journal of Biotechnology.
; 2: 307–311.
. Kamanzi AK, Schmid C, Brun R,
Kone MW, Traore D.
Antitrypanosomal and
antiplasmodial activity of medicinal
plants from Côte d’Ivoire. Journal of
Ethnopharmacology. 2004; 90:
–227.
. Duke JA, Wain KK. Medicinal plants
of the World. Computer index with
more than 85,000 entries, 1981; 3:
. Burkill HM. The useful plants of
West Tropical Africa. 2nd Edition.
Royal Botanical Gardens, Kew.
; 1:650-652.
. Adegoke AA, Iberi PA, Akinpelu DA,
Aiyegoro OA, Mboto CI. Studies on
phytochemical screening and
antimicrobial potential of Phyllanthus
amarus against multiple antibiotic
resistant bacteria. International
Journal of Applied Research in
Natural Products. 2010; 3(3): 6-12.
. Iwu MM. Handbook of African
Medicinal Plants; CRC Press Inc,
Boca Raton, Ann Arbour: London,
Tokyo. 1993. p. 49.
. Odetola AA, Akkojenu SM.
Antidiarrhoeal and gastrointestinal
potentials of the aqueous extracts of
Phyllanthus amarus
(Euphorbiaceae). Afr. J. Med. 2000;
: 119-122.
. Wang MH, Cheng YL, Meng L, Zhao
G, Mai K. Herbs of the genus
Phyllanthus amarus in the treatment
of chronic hepatitis B: Observations
with three preparations from
different geographic sites. J. Lab.
Clin. Med. 1995; 126: 350-352.
. Adeneye A A, Benebo AS, Agbaje
EO. Protective effect of the aqueous
leaf and seed extract of Phyllanthus
amarus on alcohol-induced
hepatotoxicity in rats. West Afri J.
Pharmacol. Drug Res. 2006a;
,223: 42-50.
. Adeneye AA, Amole OO, Adeneye
AK. The hypoglycemic and
hypocholesterolemic activities of the
aqueous leaf and seed extracts of
Phyllanthus amarus in mice.
Fitoterapia. 2006b; 77: 511-514.
. Sofowora EA, Isaac-Sodeye WA,
Ogunkoya LO. Isolation and
characterization of an antisickling
agent from the root of Fagara
xanthoxyloides. African medicinal
plants. Proceedings of a
Conference. Eds. Sofowora E.A.,
; p. 89-97.
. Moody JO, Ojo OO, Omotade OO,
Adeyemo AA, Olumese PE,
Ogundipe OO. Antisickling potential
of a Nigerian herbal formula
(Ajawaron HF) and the major plant
component (Cissus populnea L.
CPK). Phytotherapy Research.
; 17: 1173-1176.
. Harbone JB. Phytochemical
methods: A guide to modern
techniques of plant analysis. 3rd
Edition. Chapman and Hill, London.
; p. 279.
. Kenner DL, Yves RMD. Botanical
Medicines. A European Professional
Perspective. Paradign publications.
p. 407.
. Evans WC. Trease and Evans
Pharmacognosy. 13th edition. The
Alden Press, Oxford, London. 1989.
p. 276.
. Ekeke GI, Shode FO. The reversion
of Sickled cells by Cajanus cajan.
Planta Medica. 1985; 6: 504-507.
. Ogunyemi CM, Elujoba AA,
Durosinmi MA. Antisickling
Properties of fermented mixture of
Carica papaya Linn. and Sorghum
bicolor Linn. Moench. Journal of
Natural Products. 2008; 1:56-66
. Gbadamosi IT, Moody JO, Yekini
AD. Nutritional composition of ten
ethnobotanicals used for treatment
of anaemia in southwestern Nigeria.
European Journal of Medicinal
Plants. 2012; 2(2): 140-150.
. Igwe CU, Nwaogu LA, Ujuwondu
CO. Assessment of the hepatic
effects, phytochemical and
proximate composition of P. amarus.
African Journal of Biotechnology.
; 6(6): 728-731.