Comparative assessment of the antioxidant activity and free radical scavenging potential of different parts of Nerium indicum
Keywords:
Nerium indicum, antioxidant, flavonoids, phenolics, free radicalsAbstract
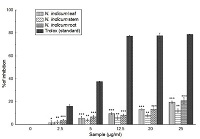
Reactive oxygen species (ROS) cause damage to cellular components. Antioxidant compounds scavenge or neutralize the ROS and thus have significant role in human health. The present study 70% methanol extracts of Nerium indicum leaf, stem and root were evaluated for in vitro total antioxidant, radical scavenging activity along with phenolic and flavonoid contents. The extracts were examined for the scavenging activity of hydroxyl radical, nitric oxide, singlet oxygen, hypochlorous acid, superoxide, peroxynitrite, hydrogen peroxide. The extracts were also tested for their potential as an iron chelating agent, inhibition of lipid peroxidation and total reducing potential. The present study indicates that the total antioxidant, DPPH (2,2-diphenyl-1-picrylhydrazyl) radical and singlet oxygen scavenging potential is in the order of stem>root>leaf. The hydroxyl radical scavenging, hydrogen peroxide scavenging and hypochlorous acid scavenging activity is in the order leaf>stem>root, whereas superoxide scavenging and lipid peroxidation inhibition assay is root>leaf>stem. Miscellaneous results were obtained in the scavenging of other radicals by the extracts, viz., leaf>root>stem for peroxynitrite and iron chelation activity, root>stem>leaf for reducing power and stem>leaf>root for nitric oxide inhibition. The phenolic and flavonoid content is in the following order root>stem>leaf and leaf>stem>root respectively. The present study revealed that the leaf, stem and root extracts of N. indicum are effective free radical scavenger and might be used as a natural source of potent antioxidant.
References
. Davies KJ. Oxidative stress: The
paradox of aerobic life. Biochem Soc
Symp. 1995;61:1–31.
. Devasagayam TPA, Tilak JC, Boloor
KK, Sane KS, Ghaskadbi S, Lele RD.
Free radicals and antioxidants in
human health: current status and
future prospects. J Assoc Physician
India. 2004;52:794–804.
. Cadenas E, Packer L. Handbook of
Antioxidants. New York. Marcel
Dekker; 1996;p. 545–591.
. Ghosh A. Ethnomedicinal plants used
in west Rarrh region of West Bengal.
Nat Pod Rad. 2008;7(5):461-465.
. Leung MY, Liu C, Koon JC.
Polysaccharide biological response
modifiers. Immunol Lett.
;105(2):101–114.
. Ding K, Fang JN, Dong T, Tsim KW,
Wu H. Characterization of a
rhamnogalacturonan and a
xyloglucan from Nerium indicum and
their activities on PC12
pheochromocytoma cells. J Nat Prod.
;66(1):7–10.
. Hussain MA, Gorsi MS. Antimicrobial
activity of Nerium oleander Linn.
Asian J Plant Sci. 2004;3:177-180.
. Abe F and Yamauchi T. Cardenolide
triosides of oleander leaves.
Phytochem. 1992;31(7):2459-2463.
. Re R, Pellegrini N, Proteggente A,
Pannala A, Yang M, Rice-Evans C.
Antioxidant activity applying an
improved ABTS radical cation
decolorization asszy. Free Rad Biol
Med. 1999;26(9-10):1231-1237.
. Mahakunakorn P, Tohda M,
Murakami Y, Matsumoto K,
Watanabe H. Antioxidant and free
radical-scavenging activity of chotosan and its related constituents. Biol
Pharm Bull. 2004;27(1):38-46.
. Hazra B, Biswas S, Mandal N.
Antioxidant and free radical
scavenging activity of Spondias
pinnata. BMC Complement Altern
Med. 2008;8:63.
. Garratt DC () The quantitative
analysis of drugs. vol 3. Japan. Chapman and Hall Ltd.; 1964;P. 456-458.
. Long LH, Evans PJ, Halliwell B.
Hydrogen peroxide in human urine:
implications for antioxidant defense
and redox regulation. Biochem
Biophys Res Commun.
;262(3):605-609.
. Beckman JS, Chen H, Ischiropulos H,
Crow JP. Oxidative chemistry of
peroxynitrite. Methods Enzymol.
;233:229-240.
. Bailly F, Zoete V, Vamecq J, Catteu
JP, Bernier JL. Antioxidant actions of
ovothiol-derived 4-
mercaptoimidazoles: glutathione
peroxidase activity and protection
against peroxynitrite-induced
damage. FEBS Lett. 2000;486(1):19-
. Pedraza-Chaverrí J, Barrera D,
Maldonado PD, Chirino YI, MacíasRuvalcaba NA, Medina-Campos ON,
Castro L, Salcedo MI, Hernán-dezPando R. S-allylmercaptocysteine
scavenges hydroxyl radical and
singlet oxygen in vitro and attenuates
gentamicininduced oxidative and
nitrosative stress and renal damage
in vivo. BMC Clin Pharmacol.
;4:5.
. Haro-Vicente JF, Martinez-Gracia C,
Ros G. Optimization of in vitro
measurement of available iron from
different fortificants in citric fruit
juices. Food Chem. 2006;98(4):639-
. Oyaizu M. Studies on products of
browning reactions: Antioxidant
activities of products of browning
reaction prepared from glucose
amine. Jap J Nutr. 1986;44:307-315.
. Kizil G, Kizil M, Yavuz M, Emen S,
Hakimoglu F. Antioxidant activities of
ethanol extracts of Hypericum
triquetrifolium and Hypericum
scabroides. Pharm Biol.
;46(4):231-242.
. Singleton VL, Rossi JA. Colorimetry
of total phenolics with
phosphomolybdic-phosphotungstic
acid reagents. Am J Enol Vitic
;16(3):144-158.
. Zhishen J, Mengcheng T, Jianming
W. The determination of flavonoid
content in mulberry and their
scavenging effects on superoxide
radicals. Food Chem.
;64(4):555-559.
. Huang D, Ou B, Prio RL. The
chemistry behind antioxidant capacity
assay. J Agric Food Chem.
;53(6):1841−1856.
. Lissi EA, Modak B, Torres R, Escobar
J, Urzua A. Total antioxidant potential
of resinous exudates from
Heliotropium species, and a
comparison of the ABTS and DPPH
methods. Free Rad Res.
;30(6):471-477.
. Packer L, Ong ASH. Biological
oxidants and antioxidants: Molecular
mechanisms and health effects.
USA. AOCS Press; 1998;p. 65–126.
. Muller FL, Lustgarten MS, Jang Y,
Richardson A, Van Remmen H.
Trends in oxidative aging theories.
Free Radic Biol Med.
;43(4):477–503.
. Miller MJ, Sadowska-Krowicka H,
Chotinaruemol S, Kakkis JL, Clark
DA. Amelioration of chronic ileitis by
nitric oxide synthase inhibition. J
Pharmacol Exp Ther. 1993;264(1):11-
. Pacher P, Beckman JS, Liaudet L.
Nitric oxide and peroxynitrite: in
Health and disease. Physiol Rev.
;87(1):315-424.
. Kochevar EI, Redmond WR.
Photosensitized production of singlet
oxygen. Method Enzymol.
;319:20-28.
. Albrich JM, McCarthy CA,Hurst JK.
Biological reactivity of hypochlorous
acid: Implications for microbicidal
mechanisms of leukocyte
myeloperoxidase. Proc Natl Acad Sci.
;78(1):210–214.
. Dennis WH, Olivieri VP, Krusé CW.
The reaction of nucleotides with
aqueous hypochlorous acid. Water
Res. 2003;13(4):357–362.
. Carr AC, Vissers MC, Domigan NM,
Winterbourn CC. Modification of red
cell membrane lipids by hypochlorous
acid andhaemolysis by preformed
lipid chlorohydrins. Redox Rep.
;3(5-6):263–71.
. Stadtman ER. Protein oxidation and
aging. Science.
;257(5074):1220–1224.
. Chang LW, Yen WJ, Huang SC, Duh
PD. Antioxidant activity of sesame
coat. Food Chem. 2002;78(3):347–
. Hertog MG, Feskens EJ, Hollman
PC, Katan MB, Kromhout D. Dietary
antioxidant flavonoids and risk of
coronary heart disease: the Zutphen
Elderly Study. Lancet.
;342(8878):1007-1011.
. Yildirim A, Mavi A, Oktay M, Kara AA,
Algur OF, Bilaloglu V. Comparison of
antioxidant and antimicrobial activities
of Tilia (Tilia argentea Desf Ex DC),
Sage (Savia triloba L.), and Black
Tea (Camellia sinensis) extracts. J
Agri Food Chem. 2000;48(10):5030-
. Dimitrios B. Sources of natural
phenolic antioxidants. Trends Food
Sci Technol. 2006;17(9):505–512.