Antioxidant and iron chelating potential of Pongammia pinnata and its role in preventing free radical induced oxidative damage in plasmid DNA
Keywords:
Oxidative stress, DNA protection, Reactive oxygen species, lipid peroxidation, phenolic contentAbstract
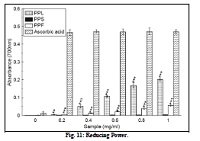
Reactive oxygen species (ROS) and free radical-mediated reactions are involved in degenerative or pathological processes. Antioxidants are believed to play an important role in preventing chronic diseases by reducing the oxidative damage to cellular components caused by ROS. In the present study, Pongamia pinnata leaf (PPL), seed (PPS), and flower (PPF) were investigated for their total phenolic and flavonoid contents, antioxidant activity by ABTS and DPPH method, scavenging activities for different free radicals such as hydroxyl, superoxide, nitric oxide, hydrogen peroxide, peroxynitrite, singlet oxygen, hypochlorous acid, the inhibition of lipid peroxidation in mice brain homogenate, reducing power, iron chelating and protection of DNA damage caused by free radicals. PPL showed the best antioxidant activity compared to both PPS and PPF. The extract of PPL possessed most potent activity compared to other extracts in scavenging assay for singlet oxygen, hydroxyl radical, superoxide radical and nitric oxide radical. PPF exhibited strongest inhibitory activity against hypochlorous acid and peroxynitrite anion among these three extracts. PPL was the best amongst three to inhibit lipid peroxidation and Fe2+-ferrozine complex formation. PPL was also found effective in protecting plasmid DNA nicking at lower concentration while both PPS and PPF did the same at higher concentration. PPL presented highest content of phenolics and flavonoids among these three extracts. The present results show that Pongammia pinnta acts as an antioxidant, iron chelator and protector of oxidative DNA damage.
References
Halliwell B, Gutteridge JMC. Free
radicals in biology and medicine. 3rd ed.
London, Oxford University Press, 1999.
Farber JL. Mechanisms of cell injury by
activated oxygen species. Environ Health
Perspect. 1994;102:17-24.
Stadtman ER. Protein oxidation and
aging. Science. 1992;257:1220-1224.
Maxwell SR. Prospects for the use of
antioxidant therapies. Drugs.
;49:345-361.
Ames SN, Shigrenaga MK, Hagen TM.
Oxidant, antioxidant and degenerative
disease of aging. Proc Nat Acad Sci.
;90:7915-7922.
Takagi A, Sekita K, Saitoh M, Kanno J.
Acute, subchronic and chronic toxicity
studies of a synthetic antioxidant, 2,2’-
isobutylidenebis(4,6-dimethylphenol) in
rats. J Toxic Sci. 2005;30:275-285.
Jaiswal SK., Dubey MK., Das S, Verma
AR, Rao CV. A comparative study on
total phenolic content, reducing power
and free radical scavenging activity of
aerial parts of Barleria prionitis. Int J
Phytomedicine. 2010;2:155-159.
Ahmad N, Fazal H, Abbasi BH, Farooq S.
Efficient free radical scavenging activity
of Ginkgo biloba, Stevia rebaudiana and
Parthenium hysterophorous leaves
through DPPH (2, 2-diphenyl-1-
picrylhydrazyl). Int J Phytomedicine.
;2:231-239.
Satyavati GV, Gupta AK, Tandon N.
Medicinal plants of India. Vol. 2. Indian
Council of Medical Research, New Delhi,
Sangwan S, Rao DV, Sharma RA. A
review on Pongamia Pinnata (L.) Pierre:
A great versatile leguminous plant. Nature
and Science. 2010;8:130-139.
Manandhar NP. Plants and people of
Nepal. USA, Timber Press, 2002.
Ambasta SP, Ramchandran K, Kashyapa
K, Chand R. The useful plants of India.
New Delhi, CSIR, 1992.
Badole SL, Bodhankar SL. Antidiabetic
activity of cycloart-23-ene-3β, 25-diol
(B2) isolated from Pongamia pinnata (L.
Pierre) in streptozotocin–nicotinamide
induced diabetic mice. Eur J Pharmacol.
;632:103-109.
Punitha R, Manohar S.
Antihyperglycemic and
antilipidperoxidative effects of Pongamia
pinnata (Linn.) Pierre flowers in alloxan
induced diabetic rats. J Ethnopharmacol.
;105:39-46.
Srinivasan K, Muruganandan S, Lal J,
Chandra S, Tandon SK, Prakash VR.
Evaluation of anti-inflammatory activity
of Pongamia pinnata leaves in rats. J
Ethnopharmacol. 2001;78:151-157.
Simonsen HT, Nordskjold JB, Smitt UW,
Nyman U, Palpu P, Joshi P, Varughese G.
In vitro screening of Indian medicinal
plants for antiplasmodial activity. J
Ethnopharmacol. 2001;74:195-204.
Baswa M, Rath CC, Dash SK, Mishra
RK. Antibacterial activity of Karanj
(Pongamia pinnata) and Neem
(Azadirachta indica) seed oil: a
preliminary report. Microbios.
;105:183-189.
Rameshthangam P, Ramasamy P. Antiviral
activity of bis (2-methylheptyl) phthalate
isolated from Pongamia pinnata leaves
against White Spot Syndrome Virus of
Penaeus monodon Fabricius. Virus Res.
;126:38-44.
Akhtar AH, Ahmad KD, Gilani SN, Nazir
A. Antiulcer effects of aqueous extracts of
Nigella sativa and Pongamia pinnata in
rats. Fitotera. 1996;67:195-199.
Vismaya, Belagihally SM, Rajashekhar S,
Jayaram VB, Dharmesh SM,
Thirumakudalu SKC. Gastroprotective
properties of Karanjin from Karanja
(Pongamia Pinnata) seeds; role as
antioxidant and H+,K+-ATPase inhibitor.
eCAM. 2010;doi:10.1093/ecam/neq027.
Essa MM, Subramanian P. Pongamia
pinnata modulates the oxidant-antioxidant
imbalance in ammonium chloride-induced
hyperammonemic rats. Fundam Clin
Pharm. 2006;20:299-303.
Shirwaikar A, Malini S, Kumari SC.
Protective effect of Pongamia pinnata
flowers against cisplatin and gentamicin
induced nephrotoxicity in rats. Ind J Exp
Biol. 2003;41:58-62.
Srinivasan K, Muruganandan S, Lal J,
Chandra S, Tandan SK, Raviprakash V,
Kumar D. Antinociceptive and antipyretic
activities of Pongamia pinnata leaves.
Phytother Res. 2003;17:259-264.
Tanaka T, Inuma M, Yuki K, Fuji Y,
Mizuno M. Flavonoids in root bark of
Pongamia pinnata. Phytochemistry.
;31:993-998.
Ghosh A, Mandal S, Banerji A, Banerji J.
A new biflavonyloxymethane from
Pongamia pinnata. Nat Prod Commun.
;5:1213-1214.
Yadav PP, Ahmad G, Maurya R.
Furanoflavonoids from Pongamia pinnata
fruits. Phytochemistry. 2004;65:439-443.
Scott PT, Pregelj L, Chen N, Hadler JS,
Djordjevic MA, Gresshoff PM. Pongamia
pinnata: an untapped resource for the biofuels
industry of the future. Bioenergy Res.
;1:2-11.
Mandal S, Hazra B, Sarkar R, Biswas S,
Mandal N. Assessment of the antioxidant
and reactive oxygen species scavenging
activity of methanolic extract of
Caesalpinia crista leaf. eCAM.
;doi:10.1093/ecam/nep072.
Hazra B, Sarkar R, Biswas S, Mandal N.
The antioxidant, iron chelating and DNA
protective properties of 70% methanolic
extract of ‘Katha’ (Heartwood extract of
Acacia catechu). J Compl Integr Med.
;7(1):article 5.
Yazdanparast R, Ardestani A. In vitro
antioxidant and free radical scavenging
activity of Cyperus rotundus. J Med Food.
;10:667-674.
Korycka-Dahl M, Richardson T.
Photogeneration of superoxide anion in
serum of bovine milk and in model
systems containing riboflavin and amino
acids. J Dairy Sci. 1978;61:400-407.
Dawson TM, Dawson VL, Snyder SH. A
novel neuronal messenger molecule in
brain: the free radical, nitric oxide. Annu
Neurol. 1992;32:297-311.
Balavoine GG, Geletti YV. Peroxynitrite
scavenging by different antioxidants. Part
: convenient study. Nitric oxide.
;3:40-54.
Kochevar EI, Redmond WR.
Photosensitized production of singlet
oxygen. Methods Enzymol. 2000;319:20-
Aruoma OI, Halliwell B, Hoey BM,
Butler J. The antioxidant action of Nacetylcysteine: Its reaction with hydrogen
peroxide, hydroxyl radical, superoxide,
and hypochlorous acid. Free Rad Biol
Med. 1989;6:593-597.
Hippeli S, Elstner EF. Transition metal
ion-catalyzed oxygen activation during
pathogenic processes. FEBS Lett.
;443:1-7.
Rice-Evans CA, Miller NJ, Paganga G.
Structure-antioxidant activity relationship
of flavonoids and phenolic acids. Free
Rad Biol Med. 1996;20:933-956.