Phytochemical Composition of the Extracts from Iresine herbstii and its Therapeutic use via Antioxidant and Cytotoxic Potential by Multiple In Vitro Assays
Keywords:
Iresine herbstii, antioxidant, radical scavenging, cytotoxicity, HeLaAbstract
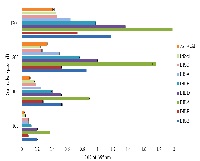
Many plants have been known to synthesize active secondary metabolites to protect themselves which have potential therapeutic applications. Iresine herbstii leaf and stem powders were extracted using ethanol, acetone, dichloromethane and petroleum ether. Phytochemicals were investigated by Gas Chromatography-Mass Spectroscopy (GCMS) and the total phenolic content as well as their therapeutic potentials also investigated by multiple in vitro assays such as radical scavenging activity, Fe3+reducing power, total antioxidant capacity and cytotoxicity towards cancer cells. Ethanolic extract was subjected for spectroscopic phytochemical analysis and therapeutic uses of I. herbstii extracts were evaluated by different in vitro methods. The total phenolic content of the samples, analyzed using Folin–Ciocalteau reagent varied from 34.67±0.58 to 103.33±1.53 mg/g dry weight, expressed as gallic acid equivalents (GAE). The 1, 1-diphenyl-2-picrylhydrazyl (DPPH) radical scavenging activity of the extracts was increased in a dose dependent manner and found that acetone extract of stem exhibited highest activity when compared to standard ascorbic acid. Cytotoxic effect of ethanolic extract of leaf was examined in vitro in HeLa cervical cancer cell line by trypan blue assay and observed over 85% reduction in live cells. The results obtained in this study clearly indicate that I. herbstii has a significant potential to be used as a natural antioxidant and anticancer agent.
References
. Jaleel CA, Gopi R, Gomathinayagam
M, Panneerselvam R. Traditional and
non-traditional plant growth regulators
alters phytochemical constituents in
Catharanthus roseus. Process
Biochem. 2009; 44 (2): 205ă209.
. Ahmed I, Mehmood Z, Mohammad F.
Screening of some Indian medicinal
plants for their antimicrobial
properties. J Ethnopharmacol. 1998;
(2): 183-193.
. Karthishwaran K, Mirunalini S,
Dhamodharan G, Krishnaveni M,
Arulmozhi V. Phytochemical
investigation of methanolic extracts of
the leaves of Pergularia daemia. J
Biol Sci. 2010; 10 (3): 242-246.
. Devasagayam TPA, Tilak JC, Boloor
KK, Sane KS, Ghaskadbi S, Lele RD.
Free radicals and antioxidants in
human health: current status and
future prospects. J Assoc Physician
India. 2004; 52:794ă804.
. Tomoko N, Takashi A, Hiromu T, Yuka
I, Hiroko M, Munekazu I, Toshiyuki T,
Tetsuro I, Fujio A, Iriya I, Tsutomu N,
Kazuhito W. Antibacterial activity of
extracts prepared from tropical and
subtropical plants on methicillin
resistant Staphylococcus aureus. J
Health Sci. 2002; 48 (3): 273-289.
. Cadenas E, Packer L. Hand Book of
Antioxidants, Plenum, New York,
; pp-203.
. Newman DJ, Cragg GM, Snader KM.
Natural products as sources of new
drugs over the period 1981-2002. J
Nat Prod. 2003; 66(7): 1022-1037.
. De Feo V. Ethnomedical field study in
northern Peruvian Andes with
particular reference to divination
practices. J Ethnopharmacol. 2003;
(2-3):243ă256.
. Bianchi A, Samorini G. Plants in
association with ayahuasca. Year
book Ethnomed. 1993; 2: 21ă42.
. Schultes RE, Hofmann A. The Botany
and Chemistry of Hallucinogens.
Charles C Thompson.
Springfield: USA 226.
. Sebold, DF. Ethnobotanical survey of
medicinal plants in the city of Campo
Bom, Rio Grande do Sul, Brazil.Porto
Alegre. Federal University of Rio
Grande do Sul. Master thesis. (2003)
. Srithi K, Balslev H,
Wangpakapattanawong P, Srisanga
P, Trisonthi C. Medicinal plant
knowledge and its erosion among the
Mien (Yao) in northern Thailand. J
Ethnopharmacol. 2009; 123 (2): 335ă
. Khare CP. Indian Medicinal Plants: An
Illustrated Dictionary. Spinger: 2007.
New York; 12.
. Vicente T, Malag´on O, Finzi PV,
Vidari G, Armijos C, Zaragoza T. An
ethnobotanical survey of medicinal
plants used in Loja and ZamoraChinchipe, Ecuador. J
Ethnopharmacol. 2007; 111 (1):63ă
. Schmidt C, Fronza M, Goettert M,
Geller F, Luik S, Flores EMM,
Bittencourtd CF, Zanettie GD,
Heinzmanne BM, Lauferb S, Merfort I.
Biological studies on Brazilian plants
used in wound healing. J
Ethnopharmacol. 2009; 122 (3): 523ă
. Cai Y, Sun M, Corke H. Antioxidant
activity of betalains from plants of the
Amaranthaceae. J Agri Food Chem.
; 51 (8): 2288î2294.
. Ebrahimzadeh MA, Pourmorad F,
Hafezi S. Antioxidant activities of
Iranian corn silk.Turkic J Biol. 2008;
: 43-49.
. Chang WC, Sei CK, Soon SH, Bong
KC, Hye JA, Min YL, Sang HP, Soo
KK. Antioxidant activity and free
radical scavenging capacity between
Korean medicinal plants and
flavanoids by assay guided
comparison. Plant Sci. 2002; 163 (6):
-1168.
. Makari HK, Haraprasad N, Patil HS,
Ravi kumar. In Vitro Antioxidant
activity of the hexane and methanolic
extracts of Cordia wallichii and
Celastrus paniculata. Internet J
Aesthet Antiaging Med. 2008; 1 (1): 1-
. Preito P, Pineda M, Aguilar M.
Spectrophotometric quantification of
antioxidant capacity through the
formation of phosphomolybdnum
complex: specific application of
vitamin E. Anal Biochem. 1999; 269
(2): 337-341.
. Slaughter JC. The naturally occurring
furanones: formation and function
from pheromone to food. Biol Rev
Camb Philos Soc. 1999; 74 (3):259-
. Harborne JB, Baxter H. The Hand
book of Natural Flavonoids. 1999.
John Wiley and Sons: Chichester.
. Johanna WL. Spicing up a vegetarian
diet: Chemopreventive effects of
phytochemicals. Amer J ClinNutr.
; 78 (3 Suppl): 579S-583S.
. Silverstein RM, Bassler GC, Morrill
TC. Spectrometric Identification of
Organic Compounds (4th Ed). John
Wiley and Sons: New York. 1981.
. Shivshankar S, Ahmad A, Sastry M.
Geranium leaf assisted biosynthesis
of silver nanoparticles. Biotechnol
Prog. 2003; 19 (6): 1627-1631.
. Jain D, Daima HK, Kachhwaha S,
Kothari SL. Synthesis of plantmediated silver nanoparticles
usingpapaya fruit extract and
evaluation of their antimicrobial
activities. Dig J Nanomaterials
Biostruct. 2009; 4 (3): 557 - 563.
. Huang J, Li Q, Sun D, Lu Y, Su Y,
Yang X, et al. Biosynthesis of silver
and gold nanoparticles by novel
sundried Cinnamomum camphora
leaf. Nanotechnol. 2007; 18 (10):
-105114.
. Song JY, Jang HK, Kim BS. Biological
synthesis of gold nanoparticles using
Magnolia kobusand Diopyros kaki leaf
extracts. Process Biochem. 2009; 44
(10): 1133ă1138.
. Shahidi F, Wanasundara PK. Phenolic
antioxidants. Crit Rev Food SciNutr.
; 32 (1): 67-103.
. Hatano T, Edamatsu R, Hiramatsu M,
Mori A, Fujita Y, Yasuhara T, Yoshida
T, Okuda T. Effects of the interaction
of tannins with co-existing
substances. VI. Effects of tannins and
related polyphenols on superoxide
anion radical and on 1, 1-diphenyl 2-
picrylhydrazyl radical. Chem Pharm
Bull. 1989; 37 (8), 2016ă2021.
. Aneta W, Jan O, Renata C.
Antioxidant activity and phenolic
compounds in 32 selected herbs.
Food Chem. 2007; 105 (3): 940ă949.
. Ayoola GA, Coker HAB, Adesegun
SA, Adepoju-Bello AA, Obaweya K,
Ezennia EC, Atangbayila TO.
Phytochemical screening and
antioxidant Activities of some selected
medicinal plants used for malaria
therapy in South western Nigeria.
Trop J Pharma Res. 2008; 7 (3):
-1024.
. Dehpour AA, Ebrahimzadeh MA,
Nabavi SF, Nabavi SM. Antioxidant
activity of methanol extract of Ferula
assafoetida and its essential oil
composition. Grasas Aceites. 2009;
(4): 405-412.
. Gordon MH, Hudson BJE. The
mechanism of the antioxidant action in
vitro. Introduction to food antioxidants.
London: Elsevier.p 1-18.
. Nabavi SM, Ebrahimzadeh MA,
Nabavi SF, Fazelian M, Eslami B. In
vitro antioxidant and free radical
scavenging activity of Diospyros lotus
and Pyrus boissieriana growing in
Iran. Phcog Mag. 2009; 4 (18): 123-
. He XG, Mocek U, Floss HG, Caceres
A, Giron L, Buckley H, Cooney G,
Manns J, Wilson BW. An antifungal
compound from Solanum nigrescens.
J Ethnopharmacol. 1994; 43 (3): 173-
. Cardellina II JH, Fuller RW, Gamble
WR, Westergaard C, Boswell J,
Munro MHG, Currens M, Boyd M.
Evolving strategies for the selection
dereplication and prioritization of
antitumorand HIV-inhibitory natural
products extracts.(999.In: Bohlin, L.,
Bruhn, J.G. (Eds.), Bioassaay
Methods in Natural Product Research
and Development. KluwerAcademic
Publishers, Dordrecht, p. 25ă36
. Kanadaswami C, Lee L, Lee PH,
Hwang J, Ke F, Huang YT, Lee MT.
The antitumor activities of flavonoids.
In Vivo. 2005; 19(5): 895-909.