The ethyl acetate fraction of Gynura procumbens sensitizes widr colon cancer cell line against 5-fluorouracil but shows antagonism with cisplatin
Keywords:
Gynura procumbens, WiDr, G1 and S phase arrest, apoptosisAbstract
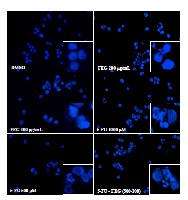
Our recent study has evaluated the ethyl acetate fraction of Gynura procumbens (FEG) as co-chemotherapeutic agent in combination with 5- fluorouracil (5-FU) and cisplatin (CISP) against WiDr colon cancer cells. This study aimed to assess whether FEG produced synergistic effect with 5- FU and CISP and to evaluate its regulation on proliferation, cell cycle, and cell death induction on WiDr colon cancer cells. (3-(4,5-dimethyl-thiazol-2-yl)-2,5-diphenyltetrazolium bromide) MTT assay was performed to determine the growth inhibitory effect of both single (FEG, 5-FU, or CISP) and combination treatments. FEG (25-500 μg/mL), 5- FU (25-1000 μM) and CISP (5-100 μM) inhibited cells growth in a dose dependent manner and exhibited an IC50 value of 125 μg/mL, 848 M and 43 M, respectively. FEG sensitized WiDr cells that was treated by 5-FU, boosting its therapeutic potential. Conversely when FEG was combined with CISP, it caused antagonism. The antiproliferative effect of single and combination treatment was determined by studying the cell proliferation kinetics using MTT assay. Flowcytometry and (4’,6-diamidino-2- phenylindole) DAPI staining was used to disclose the mechanism of cell cycle arrest and apoptosis. FEG inhibited cell proliferation, induced G1 and S phase arrest and apoptosis. The inhibitory effect was enhanced when FEG was combined with 5-FU, differing from CISP. According to the datas obtained, FEG possess sensitizing properties causes cell cycle arrest and cell death suppose to be apoptosis on WiDr cells. FEG demonstrates a possibility of additive to synergism properties when combined with 5-FU but shows antagonis with CISP.
References
. Perry LM. Medicinal plants of east and
southeast Asia: attributed properties and
uses, The MIT press, USA; 1980. p.94.
. Iskander MN, Song Y, Coupar IM,
Jiratchariyakul W. Antiinflamatory
screening of medicinal plant G.
Procumbens. Plant Foods Hum Nutr.
; 57: 233-244.
. Kim M, Lee HJ, Wiryowidagdo S, Kim
HK. Antihypertensive effects of G.
Procumbens extract in spontaneously
hypertensive Rats. J Med Food. 2006;
(4): 587-590.
. Rosidah YMF, Sadikun A, Asmawi MZ.
Antioxidant potential of Gynura
procumbens. Pharmaceutical Biol. 2008;
: 616-625.
. Sugiyanto, Sudarto, Meiyanto E, Nugroho
AE, Jenie UA. The anticarcinogenic
activity of plants compounds. Indonesian
Journal of Pharmacy. 2003; 14(4): 216-
. Meiyanto E, Susilowati S, Tasminatun S,
Murwanti R, Sugiyanto.
Chemopreventive effect of ethanolic
extract of Gynura procumbens (Lour),
Merr on the carcinogenesis of Rat breast
cancer development, Indonesian Journal
of Pharmacy. 2007; 18(3): 154-161.
. Meiyanto E, Septisetyani EP.
Antiproliferative and apoptotic effect of
fenolic fraction of ethanolic extract of
Gynura procumbens (Lour.) Merr. against
HeLa Cells. Artocarpus. 2005; 5(2): 74-
. Jenie RI, Meiyanto E, Murwanti R.
Antiangiogenic effect of sambung nyawa
leaves (Gynura procumbens (Lour.)
Merr.) etanolic extract on chick embryo
chorioallantoic membrane (CAM).
Indonesian Journal of Pharmacy. 2006;
(1): 50-55.
. Jenie RI, Meiyanto E. Co-chemotherapy
of sambung nyawa (Gynura procumbens
(Lour.) Merr.) leaves ethanolic extract and
Doxorubicin on breast cancer cell.
Indonesian Journal of Pharmacy. 2007;
(2): 81-87.
. Lee HJ, Lee BC, Chung JH,
Wiryowidagdo S, Chun W, Kim SS, Kim
H, Choe M. Inhibitory effects of aqueous
extract of Gynura procumbens on human
mesangial cell proliferation. Korean J
Physiol Pharmacol. 2007; 11: 145-148.
. Sadikun A, Idus A, Ismail N., Sterol and
sterol glycosides from the leaves of
Gynura procumbens. Nat Prod Sci. 1996;
-23.
. American Cancer Society (ACS). Cancer
Facts & Figures 2009, Atlanta: American
Cancer Society. 2009. p. 1-3.
. Davis JM, Navolanic PM, WeinsteinOppenheimer CR, Steelman LS, Wei H,
Konopleva M, Blagosklonny MV,
McCubrey JA. Raf-1 and Bcl-2 Induce
Distinct and Common Pathways That
Contribute to Breast Cancer Drug
Resistance, ClinicalCancer Research.
; 9: 1161-1170.
. Notarbartolo M, Poma P, Perri D,
Dusonchet L, Cervello M, Alessandro N.
Antitumor effects of curcumin, alone or in
combination with cisplatin or
doxorubicin, on human hepatic cancer
cells. Analysis of their possible
relationship to changes in NF-кB
activation levels and in IAP gene
expression. Cancer Letter. 2005; 224: 53-
. Cardenas EE, Sanfridson A, Culter NS,
Heitmam J. Signal-transduction cas cades
as Target for Therapeutical Intervetion by
Natural Products. Tibtech. 1998; 16: 425-
. Violette S, Poulain L, Dussaulx E, Pepin
D, Faussat AM, Chambaz J, Lacorte JM,
Staedel C, Lesuffleur T. Resistance of
colon cancer cells to long-term 5-
fluorouracil exposure is correlated to the
relative level of BCl-2 and BCl-XL in
addition to Bax and p53 status. Int J
Cancer. 2002; 98: 498-504.
. Maryati, Meiyanto E, Riyanto S. Uji
Sitotoksik dan isolasi flavonoid dari fraksi
etil asetat daun Gynura procumbens
(Lour) Merr. Pharmacon. 2005; 6(2): 46-
. Huang WW, Chiu YJ, Fan MJ, Lu HF,
Yeh HF, Li KH, Chen PY, Chung JG,
Yang JS., Kaempferol induced apoptosis
via endoplasmic reticulum stress and
mitocondria-dependent pathway in human
osteosarcoma U-2 OS cells. Mol Nutr
Food Res. 2010; 54: 1-11.
. Choi EJ, Ahn WS. Kaempferol induced
the apoptosis via cell cycle arrest in
human breast cancer MDA-MB-453 cells.
Nutr Res Prac. 20082(4), 322-325.
. Priego S, Feddi F, Ferrer P, Mena S,
Benlloch M, Ortega A, Carretero J,
Obrador E, Miguel AM, Estrela JM.
Natural polyphenols facilitate elimination
of HT-29 colorectal cancer xenografts by
chemoradiotherapy: a Bcl-2- and
superoxide dismutase 2-dependent
mechanism. Mol Cancer Ther. 2008;
(10): 3330–42.