Phytoestrogens of Pachyrhizus erosus prevent Bone Loss in an Ovariectomized Rat Model of Osteoporosis
Keywords:
phytoestrogen, Pachyrhizus erosu, ovariectomizedrat, osteoporosisAbstract
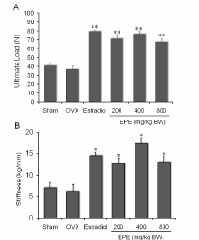
The effects of the etyl acetate extract of root of Pachyrhizus erosus (L) Urb (EPE) on bone loss and in ovariectomized (ovx) rats model of osteoporosis were investigated. Forty-two 6-weeksold female Sprague–Dawley rats were randomly assigned to six groups as followed, sham-operated, OVX, OVX-Estradiol (2 μg/day), OVX-EPE 200 mg/kg BW, OVX-EPE 400 mg/kg BW, OVX-EPE 800 mg/kg BW for 4 weeks. The administration of EPE was given orally using a stomach tube. The results demonstrated that the administration EPE 200, 400, and 800 mg/kg BW significantly prevented bone loss in OVX rats which these effect equivalent to estradiol. These effects were described in increased length of femur and tibiae, bone density, and mineral content of calcium and phosphorous in bone ash. EPE also significantly prevented OVX-induced uterine atrophy and increased in body weight gain. The femur mechanical testing significantly increased the ultimate load and stiffness of femurs of ovariectomized-rats that its effect was greater than OVX or shamoperated rats. Increased bone density may lead to enhanced bone strength, reducing the risk of fracture, which is evident in the administration of EPE due to high content of mineral density and content and increase the ultimate load. This effect seems to be pro-estrogenic compound, which suppress bone resorption by directly acting on estrogen receptor in bone sites. This study suggest that phytoestrogen compound from Pachyrhizus erosus may offer a potential alternative therapy for the treatment of health problems such as osteoporosis in post-menopausal women.
References
Maggi S, Kelsey JL, Litvak J, Heyse SP.
Incidence of hip fractures in the elderly: a
cross-national analysis. Osteoporos Int
;1:232–41.
Ross PD, Fujiwara S, Huang C, Davis JW,
Epstein RS, Wasnich RD, Kodama K,
Melton LJ Vertebral fracture prevalence in
women in Hiroshima compared to Caucasian
or Japanese in the US. Inc J Epidemiol
;24:1171–1177.
Lau EMC, Cooper C. The epidemiology of
osteoporosis: the oriental perspective in a
world context. Clin Orthop 1996; 323:65–74.
Lindsay R, Hart DM, Aitken JM, MacDonald
ED, Anderson JB, Clarke AC. Long-term
prevention of postmenopausal osteoporosis
by oestrogen. Lancet 1976;1:1038–1041.
Lindsay R, Hart DM, Clark DM. The
minimum effective dose of estrogen for
prevention of postmenopausal bone loss.
Obstet Gynecol 1984;63:759–763.
Gordon GS, Picchi J, Roof BS. Antifracture
efficacy of long-term oestrogens for
osteoporosis. Trans Assoc Am Physicians
;86: 326–331.
Hutchinson TA, Polansky SM, Feinstein AP.
Postmenopausal oestrogens protect against
fractures of hip and distal radius. Lancet
;2:706–709.
Michaëlsson K, Baron JA, Farahmand BY,
Johnell O, Magnusson C, Persson PG,
Persson I, Ljunghall S. Hormone
replacement therapy and risk of hip fracture:
population based case-control study. The
Swedish Hip Fracture Study Group. BMJ
;316:1858–1863.
Blank RD, Bockman RS. A review of clinical
trials of therapies for osteoporosis using
fracture as an end point. J Clin Densitom
;2: 435–452.
Writing Group for the Women’s Health
Initiative Investigators. Risks and benefits of
estrogen plus progestin in healthy
postmenopausal women: principal results
from the Women’s Health Initiative
randomized controlled trial. JAMA
;288:321–333.
Lacey JV Jr, Mink PJ, Lubin JH, Sherman
ME, Troisi R, Hartge P, Schatzkin A,
Schairer C. Menopausal hormone
replacement therapy and the risk of ovariacancer. JAMA 2002;288:334–341.
Arts J, Kuiper GG, Janssen JM, Gustafsson
JA, Löwik CW, Pols HA, van Leeuwen JP.
Differential expression of estrogen receptors
α and β mRNA during differentiation of
human osteoblast SV-HFO cells.
Endocrinology 1997;138:5067–5070.
Onoe Y, Miyaura C, Ohta H, Nozawa S,
Suda T. Expression of estrogen receptor β in
rat bone. Endocrinology 1997;138:4509–
Black LJ, Sato M, Rowley ER, Magee DE,
Bekele A, Williams DC, Cullinan GJ,
Bendele R, Kauffman RF, Bensch WR.
Raloxifene (LY139481 HCI) prevents bone
loss and reduces serum cholesterol without
causing uterine hypertrophy in
ovariectomized rats. J Clin Invest
;93:63–69.
Ettinger B, Black DM, Mitlak BH,
Knickerbocker RK, Nickelsen T, Genant HK,
Christiansen C, Delmas PD, Zanchetta JR,
Stakkestad J, Glüer CC, Krueger K, Cohen
FJ, Eckert S, Ensrud KE, Avioli LV, Lips P,
Cummings SR. Reduction of vertebral
fracture risk in postmenopausal women with
osteoporosis treated with raloxifene: results
from a 3-year randomized clinical trial.
JAMA 1999;282:637–645.
Pike AC, Brzozowski AM, Hubbard RE,
Bonn T, Thorsell AG, Engström O,
Ljunggren J, Gustafsson JA, Carlquist M.
Structure of the ligand binding domain of
oestrogen receptor beta in the presence of a
partial agonist and a full antagonist. EMBO J
;18:4608–4618.
Bryant HU, Glasebrook AL,Yang NN, Sato
M. An estrogen receptor basis for raloxifene
action in bone. J Steroid Biochem Mol Biol
;69:37–44.
Anderson JJB, Garner SC. The effects of
phytoestrogens on bone. Nutr Res
;20:220–224.
Messina M. Soyfoods, soybean isoflavones,
and bone health. Korean Soybean Digest
;15:122–136.
Ishida H, Uesugi T, Hirai K, Toda T, Nukaya
H, Yokotsuka K, Tsuji K. Preventive effects
of the plant isoflavones, daidzin and genistin,
on bone loss in ovariectomized rats fed a
calcium-deficient diet. Biol Pharm Bull
;21, 62–66.
Kurzer MS, Xu X. Dietary phytoestrogen.
Annu Rev Nutr 1997;17:353–381.
Mazur W, Adlercreutz H. Overview of
naturally occurring endocrineactive
substances in the human diet in relation to
human health. Nutrition 2000;16:654–658.
Arjmandi BH, Alekel L, Hollis BW, Amin
D, Stacewicz-Sapuntzakis M, Guo P,
Kukreja SC. Dietary soybean protein
prevents bone loss in an ovariectomized rat
model of osteoporosis. J Nutr 1996;126:161–
Arjmandi BH, Birnbaum R, Goyal NV,
Getlinger MJ, Juma S, Alekel L, Hasler CM,
Drum ML, Hollis BW, Kukreja S. Bonesparing effect of soy protein in ovarian
hormone-deficient rats is related to its
isoflavone content. Am J Clin Nutr 1998,
(Suppl 6):1364S–1368S.
Fanti P, Monier-Faugere MC, Geng Z,
Schmidt J, Morris PE, Cohen D, Malluche
HH. The phytoestrogen genistein reduces
bone loss in shortterm ovariectomized rats.
Osteoporos Int 1998;8:274–281.
Ishimi Y, Miyaura C, Ohmura M, Onoe Y,
Sato T, Uchiyama Y, Ito M, Wang X, Suda
T, Ikegami S. Selective effects of genistein, a
soybean isoflavone, on B-lymphopoiesis and
bone loss caused by estrogen deficiency.
Endocrinology 1999;140:1893–1900.
Picherit C, Coxam V, Bennetau-Pelissero C,
Kati-Coulibaly S, Davicco MJ, Lebecque P,
Barlet JP. Daidzein is more efficient than
genistein in preventing ovariectomy-induced
bone loss in rats. J Nutr 2000; 130:1675–
Glazier MG and Bowman MA. A review of
the evidence for use of phytoestrogens as a
replacement for traditional estrogen
replacement therapy. Arch Internal Med
; 161: 1161–1172.
Farmakalidis E, Hathcock JN, Murphy PA.
Oestrogenic potency of genistin and daidzin
in mice. Food Chem. Toxicol 1985; 23:741–
Kalu DN, Liu CC, Salerno E, Hollis B,
Echon R, Ray M. Skeletal response of
ovariectomized rats to low and high doses of
β-estradiol. Bone Miner 1991; 14:175–
Kalu DN, Masoro EJ, Byung PB, Hardin RR,
Hollis BW. Modulation of age-related
hyperparathyroidism and senile bone loss in
Fisher rats by soy protein and food
restriction. Endocrinology 1988;122:1847–
Yao W, Hadi T, Jiang Y, Lotz J, Wronski TJ,
Lane NE. Basic fibroblast growth factor
improves trabecular bone connectivity and
bone strength in the lumbar vertebral body of
osteopenic rats. Osteoporos Int
;16:1939–1947.
Lukitaningsih E: The exploration of
whitening and sun screening compounds in
bengkoang roots (Pachyrhizus erosus), Ph.D
Thesis. Wuerzburg University, Faculty of
Pharmacy and Food Chemistry; 2009.
Uesugi T, Toda T, Tsuji K, Ishida H.
Comparative study on reduction of bone loss
and lipid metabolism abnormality in
ovariectomized rats by soy isoflavones,
daidzin, genistin, and glycitin. Biol Pharm
Bull 2001; 24:368–372.