Phytochemical analysis, Antimicrobial, insecticidal and antiradical activity of Hydnocarpus pentandra (Buch.-Ham.) Oken
Keywords:
Hydnocarpus pentandra, Phytochemical, Agar well diffusion, Poisoned food technique, DPPH, ABTS, Aedes aegyptiAbstract
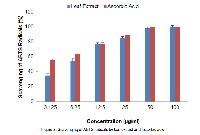
Objectives: The present study was conducted to evaluate antimicrobial, insecticidal and radical scavenging activity of leaf extract of Hydnocarpus pentandra (Buch.-Ham.) Oken belonging to the family Achariaceae. Methods: Extraction process of shade dried and powdered leaf was carried out by maceration technique. Extract was screened for phytochemicals by standard tests. Antibacterial and antifungal activity of leaf extract was determined by Agar well diffusion and Poisoned food technique respectively. Antiradical activity of leaf extract was evaluated by two in vitro assays namely 1,1-diphenyl-2-picrylhydrazyl (DPPH) and 2,2-azinobis 3-ethylbenzothiazoline 6-sulfonate (ABTS) free radical scavenging assays. Insecticidal activity of leaf extract was determined against II instar and IV instar larvae of Aedes aegypti. Results: Preliminary phytochemical analysis showed the presence of alkaloids, flavonoids, tannins, saponins, glycosides, triterpenes and steroids in the leaf extract. Leaf extract exhibited marked inhibitory activity against Gram positive bacteria when compared to Gram negative bacteria. Bacillus cereus (zone of inhibition 1.86±0.05cm) and Escherichia coli (zone of inhibition 1.06±0.05cm) were inhibited to highest and least extent respectively. Extract was effective in inhibiting mycelial growth of seed-borne fungi. Among fungi, the susceptibility to extract was in the order: Curvularia sp. (53.64% inhibition) > Fusarium sp. (45.81% inhibition) > Alternaria sp. (35.08% inhibition). The extract exhibited concentration dependent larvicidal activity with marked activity being observed against II instar larvae (LC50 value 0.79mg/ml) when compared to IV instar larvae (LC50 value 1.37mg/ml). Leaf extract scavenged DPPH and ABTS radicals dose dependently with an IC50 value of 13.91µg/ml and 6.03µg/ml respectively. Conclusions: The plant is shown to be an important source of bioactive agents. The observed bioactivities could be attributed to the phytochemicals present in the leaf extract. Further studies on characterization and bioactivity determination of isolated components from leaf extract are to be carried out.
References
. Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999; 12(4): 564-582.
. Garcia NVM, Gonzalez A, Fuentes M, Aviles M, Rios MY, Zepeda G, Rajos MG. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003; 87: 85-88.
. Saga T, Yamaguchi K. History of Antimicrobial agents and resistant bacteria. Japan Med Assoc J. 2009; 52(2): 103–108.
. Dahiya P, Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J Pharm Sci. 2012; 74(5): 443-450.
. Naz R, Bano A. Phytochemical screening, antioxidants and antimicrobial potential of Lantana camara in different solvents. Asian Pac J Trop Dis. 2013; 3(6): 480-486.
. Dantas G, Sommer MOA. How to fight back against antibiotic resistance. Am Sci. 2014; 102: 42-51.
. Brown D. Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void?. Nat Rev Drug Discov. 2015; 14(12): 821-832.
. Kekuda PTR, Akarsh S, Darshini SM, Prafulla D, Raghavendra HL. Antiradical and antimicrobial activity of Atylosia lineata Wt. And Arn. Sci Technol Arts Res J. 2015; 4(3): 180-183.
. Deising HB, Reimann S, Pascholati SF. Mechanisms and significance of fungicide resistance. Braz J Microbiol. 2008; 39(2): 286-295.
. Secor GA, Rivera VV. Fungicide resistance assays for fungal plant pathogens. Methods Mol Biol. 2012; 835: 385-392.
. Yoon M-Y, Cha B, Kim J-C. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol J. 2013; 29(1): 1-9.
. Hahn M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Biol. 2014; 7(4): 133-141.
. Kekuda PTR, Raghavendra HL, Solomon T, Duressa D. Antifungal and antiradical potential of Moringa stenopetala (Baker f.) Cufod (Moringaceae). J Biosci Agric Res. 2016; 11: 923-929.
. Gakuubi MM, Maina AW, Wagacha JM. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int J Microbiol. 2017; 2017: 8761610.
. Vinayaka KS, Swarnalatha SP, Preethi HR, Surabhi KS, Kekuda PTR, Sudharshan SJ. Studies on in vitro antioxidant, antibacterial and insecticidal activity of methanolic extract of Abrus pulchellus Wall (Fabaceae). Afr J Basic Appl Sci. 2009; 1(5-6): 110-116.
. Ciccia G, Coussio J, Mongelli E. Insecticidal activity against Aedes aegypti larvae of some medicinal South American plants. J Ethnopharmacol. 2000; 72(1-2): 185-189.
. Tedeschi P, Leis M, Pezzi M, Civolani S, Maietti A, Brandolini V. Insecticidal activity and fungitoxicity of plant extracts and components of horseradish (Armoracia rusticana) and garlic (Allium sativum). J Environ Sci Health B. 2011; 46(6): 486-490.
. Kumar S, Wahab N, Mishra M, Warikoo R. Evaluation of 15 local plant species as larvicidal agents against an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Front Physiol. 2012; 3: 104.
. Sivaraman G, Paulraj GM, Gandhi RM, Reegan DA, Ignacimuthu S. Larvicidal potential of Hydnocarpus pentandra (Buch.- Ham.) Oken seed extracts against Aedes aegypti Linn. and Culex quinquefasciatus Say (Diptera: Culicidae). Int J Pure Appl Zool. 2014; 2(2): 109-112.
. Kekuda PTR, Dileep N, Rakesh KN, Junaid S, Raghavendra HL. Elemental analysis and bioactivities of ripe and unripe pericarp of Polyalthia longifolia (Annonaceae). Sci Technol Arts Res J. 2014; 3(2): 68-75.
. Sharma A, Kumar S, Tripathi P. Evaluation of the Larvicidal efficacy of five indigenous weeds against an Indian strain of dengue vector, Aedes aegypti L. (Diptera: Culicidae). J Parasitol Res. 2016; 2016: 2857089.
. Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008; 4(2): 89-96.
. Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009; 2(5): 270-278.
. Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacog Rev. 2010; 4(8): 118-126.
. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012; 5(1): 9-19.
. Junaid S, Rakesh KN, Dileep N, Poornima G, Kekuda PTR, Mukunda S. Total phenolic content and antioxidant activity of seed extract of Lagerstroemia speciosa L. Chem Sci Trans. 2013; 2(1): 75-80.
. Naimi F, Bousta D, Balouiri M, Meskaoui AEL. Antioxidant and free radical-scavenging properties of seeds flavonoids extract of Cedrus atlantica Manetti, Linum usitatissimum L. and Ocimum basilicum L. species. J Appl Pharm Sci. 2015; 5(8): 95-99.
. George SA, Bhadran S, Sudhakar M, Harini BP. Comprehensive in vitro evaluation of pharmacological activities of selected plant extracts and gas chromatography-mass spectrometry profiling of Flacourtia jangomas flower extract. Asian J Pharm Clin Res. 2017; 10(5): 237-244.
. Bhat GK. Flora of South Kanara. Akriti Prints, Mangalore, India, 2014.
. Joshi AB, Harijan KC. Physicochemical and phytochemical investigation of the roots of Hydnocarpus pentandrus (Buch.-Ham.) Oken. Int J Pharm Sci Rev Res. 2014; 25(1): 260-265.
. Sahoo MR, Dhanabal SP, Jadhav AN, Reddy V, Muguli G, Babu UV, Rangesh P. Hydnocarpus: an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 2014; 154(1): 17-25.
. Varghese B, Sandhya S, Kavitha MP, Krishnakumar K. Genus Hydnocarpus: A review. International Journal of Phytopharmacology. 2016; 7(3): 143-154.
. Zahid IH, Bawazir AS, Naser R. Plant based native therapy for skin problems in Aurangabad district (M.S.). J Pharmacog Phytochem. 2013; 2(1): 241-244.
. Deepa MR, Dharmapal SP, Udayan PS. Floristic diversities and medicinal importance of selected sacred groves in Thrissur district, Kerala. Tropical Plant Research. 2016; 3(1): 230-242.
. Krishnan SM, Dhanalakshmi P, Sudhalakshmi YG, Gopalakrishnan S, Manimaran A, Sindhu S, Sagadevan E, Arumugam P. Evaluation of phytochemical constituents and antioxidant activity of Indian medicinal plant Hydnocarpus pentandra. Int J Pharm Pharm Sci 2013; 5(2): 453-458.
. Shirona TK, Sruthy KB, Rajendran N. Antibacterial and antioxidant properties of two medicinal plants from Kerala, India. International Journal of Chemical and Pharmaceutical Sciences. 2014; 5(1): 68-72.
. Rengaraju S, Gurunagarajan S. In vitro and in vivo anticancer activity of aqueous extract of Hydnocarpus pentandra (Buch.-Ham.) oken. against Ehrlich ascites carcinoma cell lines. Int J Pharm Bio Sci. 2017; 8(2): 964-972.
. Raghavendra HL, Kekuda PTR, Akarsh S, Ranjitha MC, Ashwini HS. Phytochemical analysis, antimicrobial and antioxidant activities of different parts of Pleocaulus sessilis (Nees) Bremek (Acanthaceae). Int J Green Pharm. 2017; 11(2): 98-107.
. Bhandary SK, Kumari S, Bhat VS, Sharmila KP, Bekal MP. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. Nitte Univ J Health Sci. 2012; 2(4): 34-38.
. Mir AM, Sawhney SS, Jassal MM. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J Pharm Pharmocol. 2013; 2(1): 1-5.
. Valgas C, de Souza SM, Smânia EFA, Smânia Jr A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007; 38: 369-380.
. Junaid S, Rakesh KN, Dileep N, Kekuda PTR. Antifungal, anthelmintic and insecticidal activity of ripe and unripe pericarp of Polyalthia longifolia (Annonaceae). Pharmanest. 2014; 5(4): 2217-2220.
. Kekuda PTR, Akarsh S, Nawaz NAS, Ranjitha MC, Darshini SM, Vidya P. In vitro antifungal activity of some plants against Bipolaris sorokiniana (Sacc.) Shoem. Int J Curr Microbiol Appl Sci. 2016; 5(6): 331-337.
. Elmastas M, Gulcin I, Isildak O, Kufrevioglu OI, Ibaoglu K, Aboul-Enein HY. Radical scavenging activity and antioxidant capacity of Bay leaf extracts. J Iran Chem Soc. 2006; 3(3): 258-266.
. Yusuf AZ, Zakir A, Shemau Z, Abdullahi M, Halima SA. Phytochemical analysis of the methanol leaves extract of Paullinia pinnata Linn. J Pharmacogn Phytother. 2014; 6: 10-16.
. Pisoschi AM, Pop A, Cimpeanu C, Predoi G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxid Med Cell Longev. 2016; 2016: 9130976.
. Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ju Y. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014; 22: 296-302.
. David T, George KV. HPTLC studies on the leaf extract of Hydnocarpus pentandra (Buch.-Ham.) Oken. International Journal of Pharmacy and Life Sciences. 2015; 6(3): 4349-4362.
. George SA, Harini BP, Bhadran S. In vitro assessment of antifungal activity of selected botanicals on Candida tropicalis. International Journal of Recent Scientific Research. 2016; 7(4): 9863-9866.
. Sivaraman G, Paulraj MG, Balakrishna K, Irudayaraj SS, Ignacimuthu S, Al-Dhabi NA. Biological effects of active fraction isolated from Hydnocarpus pentandra (Bunch. –Ham.) Oken seeds against Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae). Arch Phytopathol Plant Prot. 2017; 50(5-6): 262-274.
. Kshirsagar R, Upadhyay S. Free radical scavenging activity screening of medicinal plants from Tripura, Northeast India. Nat Prod Rad. 2009; 8(2): 117-122.
. Ashafa AOT, Grierson DS, Afolayan AJ. In vitro antioxidant activity of extracts from the leaves of Felicia muricata Thunb. an underutilized medicinal plant in the eastern cape province, South Africa. Afr J Tradit Complement Altern Med. 2010; 7(4): 296-302.
. Nikolova M. Screening of radical scavenging activity and polyphenol content of Bulgarian plant species. Pharmacog Res. 2011; 3(4): 256-259.
. Rajurkar NS, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci. 2011; 73(2): 146-151.
. Sadeghi Z, Valizadeh J, Azyzian Shermeh O, Akaberi M. Antioxidant activity and total phenolic content of Boerhavia elegans (choisy) grown in Baluchestan, Iran. Avicenna J Phytomed. 2015; 5(1): 1-9.
. Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999; 26: 1231-1237.
. Al-Rimawi F, Rishmawi S, Ariqat SH, Khalid MF, Warad I, Salah Z. Anticancer activity, antioxidant activity, and phenolic and flavonoids content of wild Tragopogon porrifolius Plant Extracts. Evid Based Complement Alternat Med. 2016; 2016: 9612490. doi:10.1155/2016/9612490.
Cowan MM. Plant products as antimicrobial agents. Clin Microbiol Rev. 1999;12(4):564-582.
Garcia NVM, Gonzalez A, Fuentes M, Aviles M, Rios MY, Zepeda G, Rajos MG. Antifungal activities of nine traditional Mexican medicinal plants. J Ethnopharmacol. 2003; 87:85-88.
Saga T, Yamaguchi K. History of Antimicrobial agents and resistant bacteria. Japan Med Assoc J. 2009; 52(2):103–108.
Dahiya P, Purkayastha S. Phytochemical screening and antimicrobial activity of some medicinal plants against multi-drug resistant bacteria from clinical isolates. Indian J Pharm Sci. 2012;74(5):443-450.
Naz R, Bano A. Phytochemical screening, antioxidants and antimicrobial potential of Lantana camara in different solvents. Asian Pac J Trop Dis. 2013;3(6):480-486.
Dantas G, Sommer MOA. How to fight back against antibiotic resistance. Am Sci. 2014;102:42-51.
Brown D. Antibiotic resistance breakers: can repurposed drugs fill the antibiotic discovery void?. Nat Rev Drug Discov. 2015; 14(12):821-832.
Kekuda PTR, Akarsh S, Darshini SM, Prafulla D, Raghavendra HL. Antiradical and antimicrobial activity of Atylosia lineata Wt. And Arn. Sci Technol Arts Res J. 2015; 4(3):180-183.
Deising HB, Reimann S, Pascholati SF. Mechanisms and significance of fungicide resistance. Braz J Microbiol. 2008;39(2):286-295.
Secor GA, Rivera VV. Fungicide resistance assays for fungal plant pathogens. Methods Mol Biol. 2012; 835:385-392.
Yoon M-Y, Cha B, Kim J-C. Recent trends in studies on botanical fungicides in agriculture. Plant Pathol J. 2013;29(1):1-9.
Hahn M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J Chem Biol. 2014;7(4):133-141.
Kekuda PTR, Raghavendra HL, Solomon T, Duressa D. Antifungal and antiradical potential of Moringa stenopetala (Baker f.) Cufod (Moringaceae). J Biosci Agric Res. 2016;11:923-929.
Gakuubi MM, Maina AW, Wagacha JM. Antifungal activity of essential oil of Eucalyptus camaldulensis Dehnh. against selected Fusarium spp. Int J Microbiol. 2017;2017:8761610.
Vinayaka KS, Swarnalatha SP, Preethi HR, Surabhi KS, Kekuda PTR, Sudharshan SJ. Studies on in vitro antioxidant, antibacterial and insecticidal activity of methanolic extract of Abrus pulchellus Wall (Fabaceae). Afr J Basic Appl Sci. 2009;1(5-6):110-116.
Ciccia G, Coussio J, Mongelli E. Insecticidal activity against Aedes aegypti larvae of some medicinal South American plants. J Ethnopharmacol. 2000;72(1-2):185-189.
Tedeschi P, Leis M, Pezzi M, Civolani S, Maietti A, Brandolini V. Insecticidal activity and fungitoxicity of plant extracts and components of horseradish (Armoracia rusticana) and garlic (Allium sativum). J Environ Sci Health B.2011;46(6): 486-490.
Kumar S, Wahab N, Mishra M, Warikoo R. Evaluation of 15 local plant species as larvicidal agents against an Indian strain of dengue fever mosquito, Aedes aegypti L. (Diptera: Culicidae). Front Physiol. 2012;3:104.
Sivaraman G, Paulraj GM, Gandhi RM, Reegan DA, Ignacimuthu S. Larvicidal potential of Hydnocarpus pentandra (Buch.- Ham.) Oken seed extracts against Aedes aegypti Linn. and Culex quinquefasciatus Say (Diptera: Culicidae). Int J Pure Appl Zool. 2014; 2(2):109-112.
Kekuda PTR, Dileep N, Rakesh KN, Junaid S, Raghavendra HL. Elemental analysis and bioactivities of ripe and unripe pericarp of Polyalthia longifolia (Annonaceae). Sci Technol Arts Res J. 2014;3(2):68-75.
Sharma A, Kumar S, Tripathi P. Evaluation of the Larvicidal efficacy of five indigenous weeds against an Indian strain of dengue vector, Aedes aegypti L. (Diptera: Culicidae). J Parasitol Res. 2016;2016:2857089.
Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008;4(2):89-96.
Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2(5):270-278.
Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacog Rev. 2010;4(8):118-126.
Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012; 5(1):9-19.
Junaid S, Rakesh KN, Dileep N, Poornima G, Kekuda PTR, Mukunda S. Total phenolic content and antioxidant activity of seed extract of Lagerstroemia speciosa L. Chem Sci Trans. 2013;2(1): 75-80.
Naimi F, Bousta D, Balouiri M, Meskaoui AEL. Antioxidant and free radical-scavenging properties of seeds flavonoids extract of Cedrus atlantica Manetti, Linum usitatissimum L. and Ocimum basilicum L. species. J Appl Pharm Sci. 2015;5(8):95-99.
George SA, Bhadran S, Sudhakar M, Harini BP. Comprehensive in vitro evaluation of pharmacological activities of selected plant extracts and gas chromatography-mass spectrometry profiling of Flacourtia jangomas flower extract. Asian J Pharm Clin Res. 2017;10(5):237-244.
Bhat GK. Flora of South Kanara. Akriti Prints, Mangalore, India, 2014.
Joshi AB, Harijan KC. Physicochemical and phytochemical investigation of the roots of Hydnocarpus pentandrus (Buch.-Ham.) Oken. Int J Pharm Sci Rev Res. 2014;25(1):260-265.
Sahoo MR, Dhanabal SP, Jadhav AN, Reddy V, Muguli G, Babu UV, Rangesh P. Hydnocarpus: an ethnopharmacological, phytochemical and pharmacological review. J Ethnopharmacol. 2014; 154(1):17-25.
Varghese B, Sandhya S, Kavitha MP, Krishnakumar K. Genus Hydnocarpus: A review. International Journal of Phytopharmacology. 2016;7(3):143-154.
Zahid IH, Bawazir AS, Naser R. Plant based native therapy for skin problems in Aurangabad district (M.S.). J Pharmacog Phytochem. 2013;2(1):241-244.
Deepa MR, Dharmapal SP, Udayan PS. Floristic diversities and medicinal importance of selected sacred groves in Thrissur district, Kerala. Tropical Plant Research. 2016;3(1): 230-242.
Krishnan SM, Dhanalakshmi P, Sudhalakshmi YG, Gopalakrishnan S, Manimaran A, Sindhu S, Sagadevan E, Arumugam P. Evaluation of phytochemical constituents and antioxidant activity of Indian medicinal plant Hydnocarpus pentandra. Int J Pharm Pharm Sci 2013;5(2):453-458.
Shirona TK, Sruthy KB, Rajendran N. Antibacterial and antioxidant properties of two medicinal plants from Kerala, India. International Journal of Chemical and Pharmaceutical Sciences. 2014;5(1):68-72.
Rengaraju S, Gurunagarajan S. In vitro and in vivo anticancer activity of aqueous extract of Hydnocarpus pentandra (Buch.-Ham.) oken. against Ehrlich ascites carcinoma cell lines. Int J Pharm Bio Sci. 2017;8(2):964-972.
Raghavendra HL, Kekuda PTR, Akarsh S, Ranjitha MC, Ashwini HS. Phytochemical analysis, antimicrobial and antioxidant activities of different parts of Pleocaulus sessilis (Nees) Bremek (Acanthaceae). Int J Green Pharm. 2017;11(2): 98-107.
Bhandary SK, Kumari S, Bhat VS, Sharmila KP, Bekal MP. Preliminary phytochemical screening of various extracts of Punica granatum peel, whole fruit and seeds. Nitte Univ J Health Sci. 2012;2(4):34-38.
Mir AM, Sawhney SS, Jassal MM. Qualitative and quantitative analysis of phytochemicals of Taraxacum officinale. Wudpecker J Pharm Pharmocol. 2013;2(1):1-5.
Valgas C, de Souza SM, Smânia EFA, Smânia Jr A. Screening methods to determine antibacterial activity of natural products. Braz J Microbiol. 2007;38:369-380.
Junaid S, Rakesh KN, Dileep N, Kekuda PTR. Antifungal, anthelmintic and insecticidal activity of ripe and unripe pericarp of Polyalthia longifolia (Annonaceae). Pharmanest. 2014; 5(4):2217-2220.
Kekuda PTR, Akarsh S, Nawaz NAS, Ranjitha MC, Darshini SM, Vidya P. In vitro antifungal activity of some plants against Bipolaris sorokiniana (Sacc.) Shoem. Int J Curr Microbiol Appl Sci. 2016; 5(6):331-337.
Elmastas M, Gulcin I, Isildak O, Kufrevioglu OI, Ibaoglu K, Aboul-Enein HY. Radical scavenging activity and antioxidant capacity of Bay leaf extracts. J Iran Chem Soc. 2006;3(3):258-266.
Yusuf AZ, Zakir A, Shemau Z, Abdullahi M, Halima SA. Phytochemical analysis of the methanol leaves extract of Paullinia pinnata Linn. J Pharmacogn Phytother. 2014;6: 10-16.
Pisoschi AM, Pop A, Cimpeanu C, Predoi G. Antioxidant capacity determination in plants and plant-derived products: A review. Oxid Med Cell Longev. 2016;2016: 9130976.
Do QD, Angkawijaya AE, Tran-Nguyen PL, Huynh LH, Soetaredjo FE, Ju Y. Effect of extraction solvent on total phenol content, total flavonoid content, and antioxidant activity of Limnophila aromatica. J Food Drug Anal. 2014; 22:296-302.
David T, George KV. HPTLC studies on the leaf extract of Hydnocarpus pentandra (Buch.-Ham.) Oken. International Journal of Pharmacy and Life Sciences. 2015;6(3):4349-4362.
George SA, Harini BP, Bhadran S. In vitro assessment of antifungal activity of selected botanicals on Candida tropicalis. International Journal of Recent Scientific Research. 2016;7(4):9863-9866.
Sivaraman G, Paulraj MG, Balakrishna K, Irudayaraj SS, Ignacimuthu S, Al-Dhabi NA. Biological effects of active fraction isolated from Hydnocarpus pentandra (Bunch. –Ham.) Oken seeds against Helicoverpa armigera (Hub.) (Lepidoptera: Noctuidae). Arch Phytopathol Plant Prot. 2017; 50(5-6):262-274.
Kshirsagar R, Upadhyay S. Free radical scavenging activity screening of medicinal plants from Tripura, Northeast India. Nat Prod Rad. 2009;8(2):117-122.
Ashafa AOT, Grierson DS, Afolayan AJ. In vitro antioxidant activity of extracts from the leaves of Felicia muricata Thunb. an underutilized medicinal plant in the eastern cape province, South Africa. Afr J Tradit Complement Altern Med. 2010; 7(4):296-302.
Nikolova M. Screening of radical scavenging activity and polyphenol content of Bulgarian plant species. Pharmacog Res. 2011;3(4):256-259.
Rajurkar NS, Hande SM. Estimation of phytochemical content and antioxidant activity of some selected traditional Indian medicinal plants. Indian J Pharm Sci. 2011;73(2):146-151.
Sadeghi Z, Valizadeh J, Azyzian Shermeh O, Akaberi M. Antioxidant activity and total phenolic content of Boerhavia elegans (choisy) grown in Baluchestan, Iran. Avicenna J Phytomed. 2015;5(1): 1-9.
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Rad Biol Med. 1999;26:1231-1237.
Al-Rimawi F, Rishmawi S, Ariqat SH, Khalid MF, Warad I, Salah Z. Anticancer activity, antioxidant activity, and phenolic and flavonoids content of wild Tragopogon porrifolius Plant Extracts. Evid Based Complement Alternat Med. 2016;2016: 9612490. doi:10.1155/2016/9612490.