Toxicological evaluation of Diakure, an antidiabetic polyherbal formulation
Keywords:
Acute toxicity, sub-acute toxicity, DiaKure, polyherbal, antidiabeticAbstract
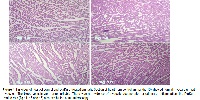
Context and Purpose of the Study: DiaKure is a hypoglycemic polyherbal formulation prepared indigenously based on knowledge of traditional medical practitioners, which contains polyherbal mixture of Vetiveria zizanioides (root), Hemidesmus indicus (rhizome), Strychnos potatorum (seed), Salacia reticulata (bark), Holarhena antidysenterica (seed), Cassia auriculata (bark), Trigonella graecum (seed) and Acacia catechu (bark) and each individual herb has scientific background in treating diabetes by the folk medical practitioners in various communities of India. The main aim of present study is to conduct an acute and sub-acute toxicological evaluation on DiaKure (an anti-diabetic polyherbal formulation), which was indigenously developed. The powder formulation is made into a decoction for better effect and easy administration. Materials and Methods: In acute toxicity tests, four groups of Wistar rats were orally treated with doses of 5, 50, 300 and 2000 mg/kg/day of DiaKure, and general behaviour, adverse effects, and mortality were recorded for up to 14 days. In sub-acute toxicity study, rats received DiaKure at the doses of 200, 500, and 1000 mg/kg/day for 28 days, and biochemical, hematological, and histopathological changes in tissues (liver, kidney, heart, and brain) were determined. Main Findings: DiaKure did not produce any signs of toxicity or mortality in the acute toxicity test. Sub-acute toxicity study with DiaKure also did not show any change in food or water consumption, hematological, or biochemical profiles. Minimal rise in body weight was noted in group III rats. Further histological study shows no necrosis or infiltration. 1000 mg/kg-treated animal showed microvesicular steatosis in individual hepatocytes. Implications: The above data showed that DiaKure could be safe for clinical use at a dose level less than or equal to 500 mg/kg. This toxicological evaluation gives this polyherbal mixture a scientific validation to the ancestral knowledge of various communities in India.
References
Gerstain HC, Santaguida P, Raina P. Morrison KM. Annual incidence and relative risk of diabetes in people with various categories of dysglycemia: a systematic overview and meta-analysis of prospective studies. Diabetes Research and Clinical Practice. 2007; 78, 305-312.
Piedrola G, Novo E, Escober F, Garcia-Robles R. White blood cell count and insulin resistance in patients with coronary artery disease. Annual Endocrinology (Paris). 2001; 62, 7–10.
Weidmann P, Boehlen LM, DE Courten M. Pathogenesis and treatment of hypertension associated with diabetes mellitus. American Heart Journal. 1993; 125, 1498–513.
Manal A. Comparative evaluation of antidiabetic activity of Rosmarinus officinalis L. and Chamomilla recutita in streptozotocin induced diabetic rats. Agriculture and Biology Journal of North America. 2012; 3, 247-52.
Efferth T, Kaina B. Toxicities by herbal medicines with emphasis to traditional Chinese medicine. Current Drug Metabolism. 2011; 12, 989-996.
Bhatt N. Ayurvedic drug industry: Challenges of today and tomorrow, Proceedings of the first national symposium of Ayurvedic drug industry, organized by ADMA, New Delhi 1998.
Sanjay Kumar Karan. Dilipkumar Pal, Sagar Kumar Mishra, and Arijit Mondal. Anti-hyperglycaemic Effect of Vetiveria zizanioides (L.) Nash Root Extract in Alloxan Induced Diabetic Rats. Asian journal of chemistry. 2013; 25, 1555-7.
Gayathri M, Kannabiran K. Hypoglycemic activity of Hemidesmus Indicus R.Br. on Streptozotocin induced diabetic rats. International Journal of Diabetes in Developing Countries. 2010; 28, 6-10.
Biswas A, Goswami TK, Ghosh A, Paul J, Banerjee K, Halder D. Hypoglycemic Effect of Strychnos Potatorum Linn were Compared with Glipizide on Male Diabetic Rats. Indian Medical Gazette. 2014; 1, 297-303.
Arunakumara KKIU, Subasinghe S. Salacia Reticulata Wight: A Review Of Botany, Phytochemistry And Pharmacology , Tropical Agricultural Research & Extension. 2010; 13, 41-47.
Jarald E, Siddheshwar B, Joshi Dharam, C Jain. Biochemical study on the hypoglycaemic effects of extract and fraction of Acacia catechu willd in alloxan-induced diabetic rats. International Journal of Diabetes & Metabolism. 2009; 17, 63-9.
Ali KM, Chatterjee K, De D Bera, TK Ghosh D. Efficacy of aqueous extract of seed of Holarrhena antidysenterica for the management of diabetes in experimental model rat: A correlative study with antihyperlipidemic activity. International Journal of Applied Research in Natural Products. 2009; 2, 13-21.
Daisy, P, Feril G, Kani J. Evaluation of Antidiabetic Activity of Various Extracts of Cassia Auriculata Linn. Bark On Streptozotocin-Induced Diabetic Wistar Rats. International Journal of Pharmacy and Pharmaceutical Sciences. 2012; 4, 312-318.
Manish Gunjan, Ravindran M, Goutam K, Jana. A Review on Some Potential Traditional Phytomedicine with Antidiabetic Properties. International Journal of Phytomedicine. 2011; 3, 448-458.
Sharma N, Sharma M, Bindal MC. Potential Antidiabetic Herbal Drugs. A Comparative Review of Marketed Products. Research Journal of Pharmacognosy and Phytochemistry. 2010; 2,115-121.
Rao MR, Palada MC, and BN Becker, Medicinal and aromatic plants in agro-forestry systems. Agroforestry Systems. 2004; 61, 107-122.
Adewunmi CO. Ojewole JAO. Safety of Traditional medicines, Complementary and Alternative Medicines in Africa. African Journal of Traditional, Complementary and Alternative Medicine. 2004; 1, 1-3.
Nasser MYA. Effect of Artemisia absinthium L. on Genotoxicity on Mice Bone Marrow Cells. World Applied Sciences Journal. 2014; 30, 770-777.
Organization for Economic Cooperation and Development (OECD).2002. Guidelines for the Testing of Chemicals /Section 4, Health Effects Test No. 423, Acute Oral toxicity - Acute Toxic Class Method.
Patrick KC, Iwuanyanwu U, Amadi IA, Charles EO, Ayalogu. Evaluation of Acute and Sub-Chronic Oral Toxicity Study of Baker Cleansers Bitters-A Polyherbal Drug on Experimental Rats. EXCLI Journal. 2012; 11, 632-40.
Organization for Economic Cooperation and Development (OECD). 2008. Guidelines For The Testing Of Chemicals 407: Repeated Dose 28-Day Oral Toxicity Study in Rodents.
Clarke ML, Clarke EGC, 1967. Garner's Veterinary toxicology. Bailliere Tindall, London.
Zbinden G, Roversi F. Significance of the LD50 test for the toxicological evaluation of chemical substances. Archives of Toxicology. 1981; 47, 77–99.
Teschke R, Frenzel C, Glass X, Schulze J, Eickhoff A. Herbal hepatotoxicity: a critical review. British Journal of Clinical Pharmacology. 2013; 75, 630–6.
Teschke R, Wolff A, Frenzel C, Schulze J. Herbal hepatotoxicity—an update on traditional Chinese medicine preparations. Alimentary Pharmacology & Therapeutics. 2014; 40, 32–50.
Firenzuoli F, Gori L. In vitro and in vivo antioxidant and toxicity evaluation of different fractions of Oxalis corniculata Linn. Journal of Pharmacological and Toxicological Methods. 2011; 6, 337-348.
Angell M, Kassierr JP. Alternative medicine – the risk of untested and unregulated remedies. N Engl J Med. 1998; 339,839-841.