Redox properties of a standardized extract of Chenopodium quinoa Willd fruit pericarp modify rat liver GST activities
Keywords:
Quinoa, GST, Thiol reducing compounds, Herbal thiolsAbstract
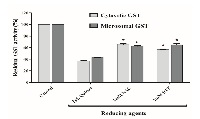
Research of antioxidant properties of herbal thiol compounds is scarce. The main non-enzymatic antioxidant compounds in animal cell are GSH and cysteine. Therefore, in this work, we studied the redox effects of a Chenopodium quinoa Willd (Quinoa) coats extract previously titrated in its polyphenol and thiol compounds. The effects of quinoa extract on rat liver cytosolic and microsomal GSH-transferase was tested. The catalytic active form of this enzyme is its disulphide dimer, and then its reduction provokes its inactivation. Quinoa extract inhibited both enzymatic activities in a concentration-dependent manner. The reducing power of this extract was significantly higher than N-acetyl-cysteine and dithiothreitol. Low concentrations of quinoa extract (without surfactant properties) decrease the apparent Vmax of both cytosolic and microsomal GST, increase their apparent GSH Km and not modify their apparent Km for substrate 1-chloro-2,4-dinitrobenzene. Redox effects of Quinoa extract would be the main cause involved in the inhibition of GSH-transferase activities. Moreover, thiol compounds present in this extract and not polyphenols seem to be the most important reducing agents acting on disulphide bond of GST active dimer. New pharmacological experiments are being carried out in order to evaluate the redox importance of thiol compounds present in this Quinoa extract.
References
R. Masella and G. Mazza, Glutathione and sulfur amino acids in human health and disease. Hoboken, New Jersey: John Wiley & Sons, Inc., 2009.
S. M. Deneke, “Thiol-based antioxidants,” Curr. Top. Cell. Regul., vol. 36, no. C, pp. 151–180, 2001.
C. G. Fraga, M. Galleano, S. V. Verstraeten, and P. I. Oteiza, “Basic biochemical mechanisms behind the health benefits of polyphenols,” Molecular Aspects of Medicine, vol. 31, no. 6. Elsevier Ltd, pp. 435–445, 2010.
F. Haber and J. Weiss, “The catalytic decomposition of hydrogen peroxide by iron salts,” Proc. R. Soc. London. Ser. A Math. Phys. Eng. Sci., vol. 147, no. 861, pp. 332–351, 1934.
H. J. H. Fenton, “Oxidation of tartaric acid in presence of iron,” J. Chem. Soc., vol. 65, pp. 899–910, 1894.
D. P. Jones, “Radical-free biology of oxidative stress.,” Am. J. Physiol. Cell Physiol., vol. 295, no. 4, pp. C849–C868, 2008.
M. Newcomb and R. E. P. Chandrasena, “Highly reactive electrophilic oxidants in cytochrome P450 catalysis,” Biochem. Biophys. Res. Commun., vol. 338, no. 1, pp. 394–403, 2005.
B. F. Coles and F. F. Kadlubar, “Detoxification of electrophilic compounds by glutathione S-transferase catalysis: determinants of individual response to chemical carcinogens and chemotherapeutic drugs?,” Biofactors, vol. 17, no. 1–4, pp. 115–30, 2003.
R. Rinaldi, E. Eliasson, S. Swedmark, and R. Morgenstern, “Reactive intermediates and the dynamics of glutathione transferases,” Drug Metab. Dispos., vol. 30, no. 10, pp. 1053–1058, 2002.
G. Wu, Y.-Z. Fang, S. Yang, J. R. Lupton, and N. D. Turner, “Glutathione metabolism and its implications for health.,” J. Nutr., vol. 134, no. 3, pp. 489–492, 2004.
H. Shen, S. Tsuchida, K. Tamai, and K. Sato, “Identification of cysteine residues involved in disulfide formation in the inactivation of glutathione transferase P-form by hydrogen peroxide.,” Archives of biochemistry and biophysics, vol. 300, no. 1. pp. 137–141, 1993.
Y. Aniya and M. W. Anders, “Activation of rat liver microsomal glutathione S-transferase by reduced oxygen species.,” J. Biol. Chem., vol. 264, no. 4, pp. 1998–2002, 1989.
M. E. Letelier, A. Molina-Berríos, J. Cortés-Troncoso, J. a. Jara-Sandoval, A. Müller, and P. Aracena-Parks, “Comparative effects of superoxide anion and hydrogen peroxide on microsomal and cytosolic glutathione S-transferase activities of rat liver,” Biol. Trace Elem. Res., vol. 134, no. 2, pp. 203–211, 2010.
M. E. Letelier, a. M. Lepe, M. Faúndez, J. Salazar, R. Marín, P. Aracena, and H. Speisky, “Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity,” Chem. Biol. Interact., vol. 151, no. 2, pp. 71–82, 2005.
M. E. Letelier, S. Sánchez-Jofré, L. Peredo-Silva, J. Cortés-Troncoso, and P. Aracena-Parks, “Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects,” Chem. Biol. Interact., vol. 188, no. 1, pp. 220–227, 2010.
H. Sies, “Polyphenols and health: Update and perspectives,” Arch. Biochem. Biophys., vol. 501, no. 1, pp. 2–5, 2010.
A. K. Parida and A. B. Das, “Salt tolerance and salinity effects on plants: a review,” Ecotoxicol. Environ. Saf., vol. 60, no. 3, pp. 324–349, Mar. 2005.
R. Repo-Carrasco, C. Espinoza, and S.-E. Jacobsen, “Nutritional Value and Use of the Andean Crops Quinoa ( Chenopodium quinoa ) and Kañiwa ( Chenopodium pallidicaule ),” Food Rev. Int., vol. 19, no. 1–2, pp. 179–189, Jan. 2003.
G. M. Woldemichael and M. Wink, “Identification and Biological Activities of Triterpenoid Saponins from Chenopodium quinoa,” J. Agric. Food Chem., vol. 49, no. 5, pp. 2327–2332, May 2001.
R. D. Reichert, J. T. Tatarynovich, and R. T. Tyler, “Abrasive Dehulling of Quinoa (Chenopodium quinoa): Effect on Saponin Content as Determined by an Adapted Hemolytic Assay,” Cereal Chem., vol. 63, no. 6. pp. 471–475, 1986.
J. M. Gee, K. R. Price, C. L. Ridout, I. T. Johnson, and G. R. Fenwick, “Effects of some purified saponins on transmural potential difference in mammalian small intestine,” Toxicol. Vitr., vol. 3, no. 2, pp. 85–90, Jan. 1989.
M. E. Letelier, C. Rodríguez-Rojas, S. Sánchez-Jofré, and P. Aracena-Parks, “Surfactant and antioxidant properties of an extract from Chenopodium quinoa Willd seed coats,” J. Cereal Sci., vol. 53, no. 2, pp. 239–243, 2011.
M. E. Letelier, A. Molina-Berríos, J. Cortés-Troncoso, J. Jara-Sandoval, M. Holst, K. Palma, M. Montoya, D. Miranda, and V. González-Lira, “DPPH and oxygen free radicals as pro-oxidant of biomolecules,” Toxicol. Vitr., vol. 22, no. 2, pp. 279–286, 2008.
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall, “Protein measurement with the Folin phenol reagent.,” J. Biol. Chem., vol. 193, no. 1, pp. 265–275, Mar. 1951.
W. Dröge, “Free radicals in the physiological control of cell function.,” Physiol. Rev., vol. 82, no. 1, pp. 47–95, 2002.
L. Migliore and F. Coppedè, “Environmental-induced oxidative stress in neurodegenerative disorders and aging,” Mutat. Res. Toxicol. Environ. Mutagen., vol. 674, no. 1–2, pp. 73–84, Mar. 2009.
I. M. Fearon and S. P. Faux, “Oxidative stress and cardiovascular disease: Novel tools give (free) radical insight,” J. Mol. Cell. Cardiol., vol. 47, no. 3, pp. 372–381, 2009.
R. Visconti and D. Grieco, “New insights on oxidative stress in cancer.,” Curr. Opin. Drug Discov. Devel., vol. 12, no. 2, pp. 240–5, Mar. 2009.
F. Q. Schafer and G. R. Buettner, “Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple,” Free Radic. Biol. Med., vol. 30, no. 11, pp. 1191–1212, Jun. 2001.
M. E. Letelier, A. Pimentel, P. Pino, A. M. Lepe, M. Faúndez, P. Aracena, and H. Speisky, “Microsomal UDP-glucuronyltransferase in rat liver: Oxidative activation,” Basic Clin. Pharmacol. Toxicol., vol. 96, pp. 480–486, 2005.