Relationship between antioxidant and anxiolytic activity of standardized extracts of Melissa officinalis and Rosmarinus officinalis
Keywords:
Melissa, Rosmarinus, officinalis, antioxidant, anxiolytic, activityAbstract
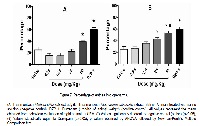
All diseases, especially those neurological pathologies (Depression, anxiety, Alzheimer and Parkinson’s diseases, etc) are described to be associated to oxidative stress. An increased concentration of metal ions in neurons of patients could be, at least in part, the cause of this oxidative stress. Reduced metal ions generate ROS through Haber-Weiss and/or Fenton reactions. By other hand, psychotropic effects of diverse plants have been described, between them Melissa officinalis and Rosmarinus officinalis. Pharmacological studies about the beneficial effects of these plants are scarce, especially those about Rosmarinus officinalis. In this work, a correlation between biological antioxidant activity of these plants and their anxiolytic activity was studied. Results show that this correlation seems to occur, and the pharmacokinetic and pharmacodynamic importance of this phenomenon is discussed.
References
I. C. K. Wong, M. L. Murray, D. Camilleri-Novak, and P. Stephens, “Increased prescribing trends of paediatric psychotropic medications.,” Arch. Dis. Child., vol. 89, no. 12, pp. 1131–1132, 2004.
World Health Organization, Pharmacological treatment of mental disorders in primary health care. World Health Organization, 2009.
N. Felicity, M. Berk, O. Dean, and A. I. Bush, “Oxidative stress in psychiatric disorders: evidence base and therapeutic implications.,” Int. J. Neuropsychopharmacol., vol. 11, no. 6, pp. 851–76, Sep. 2008.
A. Sarandol, E. Sarandol, S. S. Eker, S. Erdinc, E. Vatansever, and S. Kirli, “Major depressive disorder is accompanied with oxidative stress: Short-term antidepressant treatment does not alter oxidative - Antioxidative systems,” Hum. Psychopharmacol., vol. 22, no. 2, pp. 67–73, 2007.
M. Bilici, H. Efe, M. A. Köroǧlu, H. A. Uydu, M. Bekaroǧlu, and O. Deǧer, “Antioxidative enzyme activities and lipid peroxidation in major depression: Alterations by antidepressant treatments,” J. Affect. Disord., vol. 64, no. 1, pp. 43–51, 2001.
S. Ersan, S. Bakir, E. Erdal Ersan, and O. Dogan, “Examination of free radical metabolism and antioxidant defence system elements in patients with obsessive-compulsive disorder.,” Prog. Neuropsychopharmacol. Biol. Psychiatry, vol. 30, no. 6, pp. 1039–42, Aug. 2006.
A. Melo, L. Monteiro, R. M. F. Lima, D. M. de Oliveira, M. D. de Cerqueira, and R. S. El-Bachá, “Oxidative stress in neurodegenerative diseases: mechanisms and therapeutic perspectives.,” Oxid. Med. Cell. Longev., vol. 2011, p. 467180, Jan. 2011.
Z. J. Zhang, “Therapeutic effects of herbal extracts and constituents in animal models of psychiatric disorders,” Life Sciences, vol. 75, no. 14. pp. 1659–1699, 2004.
Y. Birdane, M. Büyüjokuroglu, F. Birdane, M. Cemek, and H. Yavuz, “Anti-inflammatory and antinociceptive effects of Melissa officinalis L. in rodents,” Rev. médecine Vet., vol. 158, no. 2, pp. 75–81, 2007.
M. E. González-Trujano, E. I. Peña, a L. Martínez, J. Moreno, P. Guevara-Fefer, M. Déciga-Campos, and F. J. López-Muñoz, “Evaluation of the antinociceptive effect of Rosmarinus officinalis L. using three different experimental models in rodents.,” J. Ethnopharmacol., vol. 111, no. 3, pp. 476–82, May 2007.
A. E. Taiwo, F. B. Leite, G. M. Lucena, M. Barros, D. Silveira, M. V Silva, and V. M. Ferreira, “Anxiolytic and antidepressant-like effects of Melissa officinalis (lemon balm) extract in rats: Influence of administration and gender.,” Indian J. Pharmacol., vol. 44, no. 2, pp. 189–92, Mar. 2012.
G. Díaz-Véliz, M. A. Vásquez, and S. Mora, “Anxiolytic and antidepressant effects of the hydroalcoholic extract of Rosmarinus officinalis (rosemary) in rats.,” in XVIII Congress of the Latin American Pharmacological Society, 2008, p. 153.
A. Ibarra, N. Feuillere, M. Roller, E. Lesburgere, and D. Beracochea, “Effects of chronic administration of Melissa officinalis L. extract on anxiety-like reactivity and on circadian and exploratory activities in mice.,” Phytomedicine, vol. 17, no. 6, pp. 397–403, May 2010.
M. E. Letelier, A. Terán, M. a. Barra, and P. Aracena-Parks, “Antioxidant properties of Rosmarinus officinalis and its effects on xenobiotic biotransformation,” Bol. Latinoam. y del Caribe Plantas Med. y Aromat., vol. 8, no. 6, pp. 487–497, 2009.
P. V Turner, T. Brabb, C. Pekow, and M. a Vasbinder, “Administration of substances to laboratory animals: routes of administration and factors to consider,” J Am Assoc Lab Anim Sci, vol. 50, no. 5, pp. 600–613, 2011.
Committee on Guidelines for the Use of Animals in Neuroscience and Behavioral Research (National Research Council), Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research. Washington DC, 2003.
M. E. Letelier, S. Sánchez-Jofré, L. Peredo-Silva, J. Cortés-Troncoso, and P. Aracena-Parks, “Mechanisms underlying iron and copper ions toxicity in biological systems: Pro-oxidant activity and protein-binding effects,” Chem. Biol. Interact., vol. 188, no. 1, pp. 220–227, 2010.
O. H. Lowry, N. J. Rosebrough, A. L. Farr, and R. J. Randall, “Protein measurement with the Folin phenol reagent.,” J. Biol. Chem., vol. 193, no. 1, pp. 265–275, Mar. 1951.
M. E. Letelier, A. Molina-Berríos, J. Cortés-Troncoso, J. Jara-Sandoval, M. Holst, K. Palma, M. Montoya, D. Miranda, and V. González-Lira, “DPPH and oxygen free radicals as pro-oxidant of biomolecules,” Toxicol. Vitr., vol. 22, no. 2, pp. 279–286, 2008.
F. Haber and J. Weiss, “The Catalytic Decomposition of Hydrogen Peroxide by Iron Salts,” Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences, vol. 147, no. 861. pp. 332–351, 1934.
H. J. H. Fenton, “Oxidation of tartaric acid in presence of iron,” J. Chem. Soc., vol. 65, pp. 899–910, 1894.
M. E. Letelier, a. M. Lepe, M. Faúndez, J. Salazar, R. Marín, P. Aracena, and H. Speisky, “Possible mechanisms underlying copper-induced damage in biological membranes leading to cellular toxicity,” Chem. Biol. Interact., vol. 151, no. 2, pp. 71–82, 2005.
M. E. Letelier, M. Faúndez, J. Jara-Sandoval, A. Molina-Berríos, J. Cortés-Troncoso, P. Aracena-Parks, and R. Marín-Catalán, “Mechanisms underlying the inhibition of the cytochrome P450 system by copper ions.,” J. Appl. Toxicol., vol. 29, no. 8, pp. 695–702, 2009.
S. Pellow, P. Chopin, S. E. File, and M. Briley, “Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat,” J. Neurosci. Methods, vol. 14, pp. 149–167, 1985.
S. Pellow and S. E. File, “Anxiolytic and anxiogenic drug effects on exploratory activity in an elevated plus-maze: a novel test of anxiety in the rat.,” Pharmacol. Biochem. Behav., vol. 24, no. 3, pp. 525–9, Mar. 1986.
A. Ibarra, N. Feuillere, M. Roller, E. Lesburgere, and D. Beracochea, “Phytomedicine Effects of chronic administration of Melissa officinalis L . extract on anxiety-like reactivity and on circadian and exploratory activities in mice,” Phytomedicine, vol. 17, no. 6, pp. 397–403, 2010.
E. Guney, M. Fatih Ceylan, A. Tektas, M. Alisik, M. Ergin, Z. Goker, G. Senses Dinc, O. Ozturk, A. Korkmaz, S. Eker, M. Kizilgun, and O. Erel, “Oxidative stress in children and adolescents with anxiety disorders,” J. Affect. Disord., vol. 156, pp. 62–66, 2014.
S. Rivera-Mancía, I. Pérez-Neri, C. Ríos, L. Tristán-López, L. Rivera-Espinosa, and S. Montes, “The transition metals copper and iron in neurodegenerative diseases.,” Chem. Biol. Interact., vol. 186, no. 2, pp. 184–99, Jul. 2010.
J. Bouayed, H. Rammal, C. Younos, and R. Soulimani, “Positive correlation between peripheral blood granulocyte oxidative status and level of anxiety in mice,” Eur. J. Pharmacol., vol. 564, no. 1–3, pp. 146–149, Jun. 2007.
R. M. McKernan, T. W. Rosahl, D. S. Reynolds, C. Sur, K. a Wafford, J. R. Atack, S. Farrar, J. Myers, G. Cook, P. Ferris, L. Garrett, L. Bristow, G. Marshall, a Macaulay, N. Brown, O. Howell, K. W. Moore, R. W. Carling, L. J. Street, J. L. Castro, C. I. Ragan, G. R. Dawson, and P. J. Whiting, “Sedative but not anxiolytic properties of benzodiazepines are mediated by the GABA(A) receptor alpha1 subtype.,” Nat. Neurosci., vol. 3, no. 6, pp. 587–592, 2000.