Antioxidant and antibacterial activity of root and seed extracts of Achyranthes aspera
Keywords:
Achyranthes aspera, ethanolic and aqueous extracts, antioxidant, antibacterial activity.Abstract
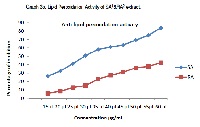
Achyranthes aspera L., a common weed belonging to Amaranthaceae family, possessing many Ayurvedic, Unani-Tibbi, Homeopathic, Siddha, Naturopathic, medicinal propertiesis widely distributed throughout the tropical world. The objective of the present study is to extract and quantify the polyphenols and flavonoids besides evaluating their antioxidant and antimicrobial activity against eight bacterial reference strains;Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Proteus vulgaris (ATCC 6380), Salmonella typhimurium (ATCC 25241), Salmonella paratyphi (ATCC 9150), Shigella sonnei (ATCC 25931) and Klebsiella pneumonia (ATCC 27736). The polyphenols and flavanoids of the extracts from both the tissues were quantified separately by Folin-Ciocalteu and Colorimetric methods respectively. The total content of antioxidants of these extracts was assessed separately by the method of Phospho-molybdenum assay, while the antioxidant activity of these extracts was evaluated by a) 1,1-Diphenyl-2-picryl- hydrazyl (DPPH) and b) lipid peroxidation methods. Further, the antibacterial activity of these extracts was evaluated separately by using agar well diffusion assay against the above mentioned eight bacterial reference strains. All the extracts showed the presence of phytochemicals; tannins, terpenoids, steroids, flavonoids and carbohydrates. The polyphenol and flavonoid content of the extracts was found to decrease in the order of seed ethanol > root ethanol>seed aqueous>root aqueous whereas the antioxidant capacity of the extracts was found to decrease in the order of seed ethanol > root ethanol>seed aqueous>root aqueous. The seed ethanol and root ethanol high DPPH scavenging activity and lipid peroxidation activity compared to seed and root aqueous. The result of this study proves that extracts are good source of antioxidants and antimicrobial agents to use them as a broad spectrum of investigation in medicines.
References
Butler MS., The role of natural product in chemistry in drug discovery. J. Nat. Prod. 2004; 67: 2141–2153.
Research Guidelines for Evaluating the Safety and Efficacy of Herbal Medicines. Manila, WHO Regional Office for the Western Pacific, (1993).
Guidelines for the Assessment of Herbal Medicines. Geneva, World Health Organization, (1991).
Mazid M, Khan TA, Mohammad F. Role of secondary metabolites in defense mechanisms of plants. Biology and Medicine. 2011; 3(2): 232-249.
Cragg GM and Newman DJ. Drug Discovery and Development from Natural Products: The Way Forward Gordon. In 11th NAPRECA Symposium Book of Proceedings, Antananarivo, Madagascar. 2005: 56-69.
Marderosian DA and Beutler JA. The Review of Natural Products. 2nd ed. Seattle, WA, USA: Facts and Comparisons; 2002. pp. 13–43.
Emori TC and Gaynes R. An overview of nosocomial infections including the role of the microbiology laboratory. Clinical Microbial Review.1993; 6(4): 428-442.
Schafer H, Wink M. Medicinally important secondary metabolites in recombinant microorganisms or plants: progress in alkaloid biosynthesis. Biotechnology Journal. 2009; 4(12): 1684-1703.
Clark AM, Hufford CD. Discovery and Development of Novel Prototype Antibiotics for Opportunistic Infections Related to Acquired Immunodeficiency Syndrome. In: Kinghorn AD and Balandrin MF, editors. Human Medicinal Agents from Plants. Vol 534. American Chemical Society; 1993. pp. 228-241.
Prior RL. Fruits and vegetables in the prevention of cellular oxidative damage. American Journal of Clinical Nutrition. 2003; 78: 570S-578S.
Narayana RK, Reddy MS, Chaluvadi MR, Krishna DR. Bioflavonoids Classification, Pharmacological, Biochemical Effects and Therapeutic Potential. Indian Journal of Pharmacology. 2001; 33: 2-16.
Dixon RA, Dey PM, Lamb CJ. Phytoalexins: enzymology and molecular biology. Adv Enzymol. 1983; 55: 1–69.
Liscovitch M, Lavie Y. Cancer multidrug resistance: a review of recent drug discovery research. IDrugs. 2002; 5: 349–355.
Lizcano LJ, Bakkali F, Ruiz-Larrea MB. Antioxidant activity and polyphenol content of aqueous extracts from Colombian Amazonian plants with medicinal use. Food Chem. 2010; 119: 1566–1570.
Hossain MA, Rahman SMM. Total phenolics, flavonoids and antioxidant activity of tropical fruit pineapple. Food Res. Int. 2011; 44: 672–676.
Gonclaves S, Gomes D, Costa P, Roman A. The phenolic content and antioxidant activity of infusions from Mediterranean medicinal plants. Industrial crop and products. 2013; 43: 465-471.
Liu RH. Health benefits of fruit and vegetables are from additive and synergistic combinations of phytochemicals. Am. J. Clin. Nutr. 2003; 78: 517S–520S.
Djeridane A, Yousfi M, Nadjemi B, Boutassouna B, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006; 97: 654–660.
Pereira RP, Fachinetto R, de Souza Prestes A, Puntel RL, Santos da Silva GN, Heinzmann BM, Boschetti TK, Athayde ML, Bürger ME, Morel AF, Morsch VM, Rocha JB. Antioxidant effects of different extracts from Melissa officinalis, Matricaria recutita and Cymbopogon citratus. Neurochem. Res. 2009; 34: 973-983.
Sharma AK., Medicinal properties of apamarg (achyranthes aspera linn.). Int. J. Ayur. Pharma Research. 2013; 1(3): 4-12.
Varuna KM, Khan MU, Sharma PK. Review on Achyranthes Aspera. Journal of Pharmacy Research. 2010; 3(4):714-717.
Sofowora A. Medicinal plants and Traditional Medicine in Africa. Ibadan: Spectrum Books; 1993. p. 150.
Trease GE and Evans WC. Pharmacognosy. 13th edn. London: Bailliere Tindall; 1989. pp. 176-180.
Ainsworth EA, Gillespie KM. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat Protoc. 2007; 2(4): 875-7.
Braca A, Sortino C, Politi M, Morelli I, Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol. 2002; 79: 379-381.
Prieto P, Pineda M & Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenum complex: specific application to determination of vitamin. Anal. Biochem.1999; 269: 337-341.
Brand WW, Cuvelier ME & Berset C. Use of free radical method to evaluate Antioxidant activity. Lebensmittel Wisssenschaft and Technologies. 1995; 28: 25-30.
Halliwell B & Gutteridge JMC. Protection against lipid peroxidation. In: Free radicals in biology and medicin. 2nd ed. Tokyo, Japan: Japan Scientific Societies Press; 1989.
Reeves DS. Antibiotic assays. In: Hawkey PM, Lewis DA, editors. Medical Bacteriology: A Practical Approach. Oxford: IRL Press; 1989. p. 195–221.
Nagavani V, Rao TR. Evaluation of antioxidant potential and qualitative analysis of major polyphenols by RPHPLC in Nymphaea Nouchali Brum Flowers. International Journal of Pharmacy and Pharmaceutical Sciences. 2010; 2(4): pp. 98-104.
Hakiman M, Maziah M. Non enzymatic and enzymatic antioxidant activities in aqueous extract of different Ficus deltoidea accessions. Journal of Medicinal Plants Research. 2009; 3(3): pp.120-131.
Odabasoglu F, Aslan A, Cakir A, Suleyman H, Karagoz Y, Halici M, Bayir Y. Comparison of antioxidant activity and phenolic content of three lichen species. Phytother. Res. 2004; 18 (11): pp.938-41.