Phytochemical screening, antioxidant activity and antimicrobial activity of Spathodeacampunalata Stem extracts.
Keywords:
Spathodea campunalata, extracts, phytochemical screening, DPPH, lipid peroxidation, antibacterial activityAbstract
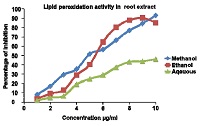
Spathodeacampunalata is African tulip tree, known for its medicinal properties, in the present study three extracts of stem Ethanolic, Methanolic and Aqueous extracts were evaluated for phenolic content, flavanoid content, total antioxidant capacity by Folin Ciocalteu method ,Colorimetric method, phosphomolybdenum assay respectively.Iinvitro antioxidant activity was evaluated by 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) radical method and by lipid peroxidation method. The antimicrobial activity was evaluated using agar well diffusion assay protocol against eight bacterial reference strains, Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), Pseudomonas aeruginosa (ATCC 27853), Proteus vulgaris (ATCC 6380), Salmonella typhimurium (ATCC 25241), Salmonella paratyphi (ATCC 9150), Shigella sonnei (ATCC 25931) and Klebsiella pneumonia (ATCC 27736). Plant sources showing antioxidant activities which is safe has become growing interest across the world thus the above experiment was performed to screen the presence of phytochemicals, estimation of polyphenols, flavonoids, and their ability to show the effects of antimicrobial and antioxidant activities was investigated. Results revealed the presence of phytoconstituents, with relevant quantity of polyphenol and flavonoid content of the extracts was found to decrease in the order of Methanolic extract > ethanolic extract>aqueous extract whereas total antioxidant activity evaluated by phosphomolybdenum assay showed decrease in the order of aqueous extract>methanolic extract>ethanolic extract. The methanolic and ethanolic extracts exhibited better activity against eight bacterial strains used compared to the aqueous extract. Invitro antioxidant activity evaluated by DPPH assay of the extracts showed concentration dependent percentage inhibition of different radicals and raised gradually to its maximum level with higher concentrations, in the lipid peroxidation, aqueous extracts shows less percentage inhibition as compared to methanolic and ethanolic extracts. Thus extract possesses significant antioxidant activity.
References
Owolabi J, Omogbai EK and Obasuyi O. Antifungal and antibacterial activities of the ethanolic and aqueous extract of kigelie Africana. (Bignoniaceae/ Stem bark). Afr. J. Biotechnol. 2007; 6(14): 882-85.
Sarker SD and Nahar L. Chemistry for Pharmacy Students General, Organic and Natural Product Chemistry. England: John Wiley and Sons; 2007. pp 283-359.
Dubey NK, Kumar R, Tripathi P. Global promotion of herbal medicine: India’s opportunity. Current Science. 2004; 86: 37–41.
Kalia AN. Text Book of Industrial Pharmacognosy. Oscar publication; 2005.
Okigbo RN, Anuagasi CL and Amadi JE. Advances in selected medicinal and aromatic plants indigenous to Africa. J. Med. Plants Res.2009; 3(2): 86 – 95.
Joy PP, Thomas J, Mathew S and Skaria. Medicinal Plants. In: Bose TK, Kabir J, Das P and Joy PP, editors. Tropical Horticulture, Vol. 2. Calcutta, India: Naya Prokash; 2001, pp: 449-632.
Valko M, Rhodes CJ, Moncol J, Izakovic M and Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem. Biol. Interact. 2006; 160: 1-40.
Gupta VK, Sharma S K. Plants as natural antioxidants. Natural Product Radiance. 2006; 5 (4): 326-334.
Percival M. Antioxidants. Clinical Nutrition Insights. 1998; (NUT031 1/96 Rev. 10/98).
Rathore GS, Suthar M, Pareek A and Gupta RN. Nutritional antioxidants: A battle for better health. J Nat Pharmaceuticals. 2011; 2: 2–14.
Kähkönen MP, Hopia AI, Vuorela HJ, Rauha J, Pihlaja K, Kujala TS and Heinonen M. Antioxidant activity of plant extracts containing phenolic compounds. J Agric Food Chem. 1999; 47: 3954–62.
Burton GW, Ingold KU. Beta-carotene - an unusual type of lipid antioxidant. Science. 1984; 224: 569–573.
Vimala S, Adenan ML, Ahmad AR and Shahdan R. Nature’s choice to wellness: antioxidant vegetables/ulam. Malaysia. Forest Research Institute; 2003.
Sharififar F, Nudeh-Dehghn G, Mirtajaldini M. Major flavonoids with antioxidant activity from Teucrium polium L. Food Chem. 2008; 112: pp.885–888.
Olaleye MT. Cytotoxicity and antibacterial activity of Methanolic extract of Hibiscus sabdariffa. J. Med. Plants Res. 2007; 1(1): 009-013.
Grassmann J, Hippeli S, Elstner EF. Plant’s defence and its benefits for animals and medicine: role of phenolics and terpenoids in avoiding oxygen stress. Plant Physiol. Biochem. 2002; 40: 471-478.
Nayak BS, Raju SS, Orette FA and Rao AV. Effects of Hibiscus rosasinensis L (Malvaceae) on Wound Healing Activity: A Preclinical Study in a Sprague Dawley Rat. Int. J. Low Extrem. Wounds. 2007; 6(2): 76- 81.
Okwu DE and Josiah C. Evaluation of the chemical composition of two Nigerian medicinal plants. Afri. J.Biotechnol. 2006; 5(4): 357-361.
Laguerre M, Lecomte J and Villeneuve P. Evaluation of the ability of antioxidants to counteract lipid oxidation: Existing methods, new trends and challenges. Progress in Lipid Research. 2007; 46(5): 244-282.,
Omonkhelin J. Owolabi, Eric KI, Omogbai and Osahon Obasuyi. Antifungal and antibacterial activities of the ethanolic and aqueous extract of Kigelia africana (Bignoniaceae) stem bark. African Journal of Biotechnology. 2007; 6 (14): 1677-1680.
SÁNCHEz-MORENO.C. Review: Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int. 2002; 8: 121-137.
Maryam Z, Farrukh A, Iqbal A. The in vitro Antioxidant activity and total phenolic content of four Indian medicinal plants. International Journal of Pharmacy and Pharmaceutical Sciences. 2009; 1(1): 88-95.
Cai Y, Luo Q, Sun M and Corke H. Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sci. 2004; 74: 2157-2184.
Dragland S, Senoo H, Wake K, Holte K and Blomhoff R. Several culinary and medicinal herbs are important sources of dietary antioxidants. J Nutr. 2003;133: 1286–90.
Brewer MS. Natural antioxidants: Sources, compounds, mechanisms of action and potential applications. Comprehensive Rev. Food Sci. Food Safety. 2011; 10: 221-247.
Jin Dai and Russell, Plant Phenolics: Extraction, Analysis and Their Antioxidant and Anticancer Properties. Molecules. 2010; 15(10); 7313-7352.
Ngouela S, Tsamo E, Sondengam BL, Connolly JD. Spathodol, A new polyhydroxysterol from the leaves of Spathodea campanulata. J Nat Prod. 1991; 54: 873-6.;
Dhanabalan R, Doss A, Jagadeeswari M, Karthic R, Palaniswamy M, ngayarkanni J. Preliminary Phytochemical Screening and Antimalarial Studies of Spathodea campanulata Leaf Extracts. Ethnobotanical Leaflets.2008;12: 811-19.
El-Hela AA. A new iridoid glucoside from Spathodea campanulata leaves. Al- Azhar Journal of Pharmaceutical Sciences. 2001b; 27:115-20.
Ngouela S, Nyasse B, Tsamo E, Sondengam BL, Connolly JD. Spathodic acid: A triterpene acid from the stem bark of Spathodea campanulata. Phytochemistry. 1990; 29: 3959-61.
Amusan OOG, Adesogan EK, Makinde JM. Antimalarial active principles of Spathodea campanulata stem bark. Phytother Res. 1996; 10: 692-3.
Subramanian SS, Sulochana N, Nagarajan S. Caffeic acid from the leaves of Spathodea campanulata. Current Science. 1973; 42: 403.
Elusiyan CA, Ani NC, Adewunmi CO, Olugbade TA. Distribution of Iridiod Glucosides and Anti-Oxidant Compounds in Spathodea campanulata Parts. Afr J Tradit Complement Altern Med. 2011; 8: 27–33.
Mbosso EJ, Ngouela S, Nguedia JC, Penlap V, Rohmer M, Tsamo E. Spathoside, a cerebroside and other antibacterial constituents of the stem bark of Spathodea campanulata.Nat Prod Res. 2008; 22: 296-304.
Ngouela S, Tsamo E, Sondengam BL. Extractives from Bignoniaceae: constituents of the stem bark of Spathodea campanulata. Planta med.1998; 54: 476.
Harborne JB. Phytochemical methods : a guide to modern techniques of plant analysis London ; New York : Chapman and Hall, 1998.
Trease GE and Evans WC. A Text-book of Parmacognosy, 14th Edn, Bailliere Tinall Ltd, London, 1989, pp. 53.
Yang D, Wang Q, Ke L, Jiang J, Ying T (2007). Antioxidant activities of various extracts of lotus (Nelumbo nucifera Gaertn.) rhizome. Asia Pac J Clin Nutr 16:158-163
Braca A, Sortino C, Politi M, Morelli I & Mendez J. Antioxidant activity of flavonoids from Licania licaniaeflora. J Ethnopharmacol, 2002; 79: 379-381.
Prieto P, Pineda M and Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of phosphomolybdenumcomplex: specific application to determination of vitamin. Anal. Biochem. 1999; 269: 337-341.
Brand WW, Cuvelier ME and Berset C. Use of free radical method to evaluate Antioxidant activity. Lebensmittel Wisssenschaft and Technologies. 1995; 28: 25-30.
Halliwell B and Gutteridge JMC. Protection against lipid peroxidation. In: Freeradicals in biology and medicin. 2nd ed. Tokyo,Japan: Japan Scientific Societies Press; 1989.
Reeves DS. Antibiotic assays. In: Hawkey PM, Lewis DA, editors. Medical Bacteriology: A Practical Approach. Oxford: IRL Press; 1989. p.195–221.
Aderogba MA, Okoh EK, Idowu TO: Evaluation of the antioxidant activity of the secondary metabolites from Pilostigma reticulatum(DC.). Hochst Biol Sci. 2005; 5: 239-242.
Harbone JB and Williams CA. Advances in flavonoid research since 1992. Phytochem. 200; 55: 481-504.
Chattopadhyay D, Maiti K, Kundu AP, Chakraborty MS, Bhadra R, Maudal SC and Maudal AB. Antibacterial activity ofAlstonia macrophylla: A folklore of bay island. J. Ethnophamacol. 2001; 77: 49-55.
Chen CW, Ho CT. Antioxidant properties of polyphenols extracted from green and black tea. Journal of Food Lipids. 1995; 2: 35-46.
Gutteridge JMC. Age pigments and free radicals: fluorescent lipid complexes formed by iron and copper containing proteins. Biochim Biophys Acta. 1985; 834: 144.
Govindarajan R, Vijayakumar M, Pushpangadan P. Antioxidant approach to disease management and the role of 'Rasayana' herbs of Ayurved. J. Ethnopharmacol. 2005; 99:165-178.
Graf E, Mahoney JR, Bryant RG, Eaton JW. Iron-catalyzed hydroxyl free radical formation: stringent requirement for iron co-ordination site. Journal Bio Chem. 1984; 259: 3620-3623.
Braugghler JM, Duncan CA, Chase LR. The involvement of iron in lipid peroxidation. Importance of ferric to ferrous ratio in initiation. J Biol Chem. 1986; 261: 10282-89.
Charami MT, Lazari D, Karioti A, Skaltsa H, Hadjipavlou-Litina D and Souleles C. Antioxidant and antiinflammatory activities of Sideritis perfoliata subsp. perfoliata (Lamiaceae). Phytotherapy Research. 2008; 22(4): p.450-4.
LAUGHTON MJ, Halliwell B, Evans PJ, Hoult JR. Antioxidant and prooxidant actions of plant phenolics quercetin, gossypol and myricetin. Effects on lipid peroxidation, hydroxyl radical generation and bleomycin dependant DNA damage. Biochemical Pharmacology. 38(17); 1989: p.2859-65.
Afanas'ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radical scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochememical Pharmacology. 1989;.38(11): p.1763-9.