Plasma Electrophoresis and Phagocytic Index Screening of Some Indigenous Vegetables Subjected to Preclinical Models
Keywords:
Colocasia esculenta, Moringa oliefera, Luffa cylindrica, Hibiscus esculentusAbstract
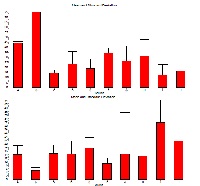
50 % of methanolic extract Colocasia esculenta, Moringa oliefera, Luffa cylindrica, and Hibiscus esculentus were subjected to immunomodulatory activity in Swiss albino mice either sex. Mice were treated with five days of dosing of Colocasia esculenta 50 mg/kg bw, Colocasia esculenta 100 mg/kg bw, Moringa oliefera 200mg/kg bw, Moringa oleifera 400 mg/kg bw, Luffa cylindrica 100 mg/kg bw, Luffa cylindrica 200 mg/kg bw, Hibiscus esculentus 100 mg/kg bw, and Hibiscus esculentus 200 mg/kg bw. Cyclosporine (2.5 mg/kg) used as a standard reference drug for 5 days. Investigation of immunomodulator activity of these 50 % of methanolic extract of drugs to parentage of yeast digestion form 24 hours the peritoneal fluid culture and electrophoretic plasma protein band albumin, alpha 1, alpha 2, beta and gamma respectively from blood plasma were observed using parameters phagocytosis and plasma electrophoresis. Also investigated the ulcerogenic effect or any toxic effect of plant extract by histopathology study of crypt, villi and goblet cells with reference to standard drug cyclosporine. As regards these parameters, Hibiscus esculentus 100 and 200 mg/ kg bw dose, Moringa oleifera 200 and 400 mg/ kg bw dose and Luffa cylindrica 200mg/kg bw elicited a moderately significant increase in the % of yeast digestion (P < 0.001) respectively and Luffa cylindrica 100mg/kg bw significant increase in the % of yeast digestion (P < 0.01). Hibiscus esculentus showed significant dose dependent increase and Moringa oleifera decrease phagocytic activity of macrophages. Hibiscus esculentus 200 mg/ kg bw dose and Moringa oleifera 200 significantly increased (P < 0.01) the Gamma globulin. However, our present study revealed and signatured for their immunomodulator enhancing property. As in Asian subcontinent daily there vegetables are cooked and served with know and unknown of its potential function against different diseases. If there vegetables properly ruled out for their pharmacological aspect then it may add diamond in the crown of dietician which has been bother every day today life but over looked exponetentially
References
Tiwari Umesh, Rastogi Bhawna, Singh
Paramjit, Saraf K Dinesh, Vyas P Suresh.
Immunomodulatory effects of aqueous extract
of Tridax procumbens in experimental
animals. Journal of Ethnopharmacology
;92:113–119.
Atal CK, Sharma ML, Kaul A, Khajuria A.
Immunomodulating agents of plant origin.
Preliminary screening: Journal of
Ethnopharmacology 1986;41:185–192.
Wagner H. Immunomodulatory agents. In:
Proceedings of the Alfred Benzon
Symposium 1983; 20: P. 559.
Desai R Veena, Ramkrishnan Rupal,
Chintalwar J Gajanan, Sainis B K. G1-4A,
an immunomodulatory polysaccharide from
Tinospora cordifolia, modulates macrophage
responses and protects mice against
lipopolysaccharide induced endotoxic shock.
International Immunopharmacology
;7:1375–1386.
P Thejass, Kuttan G. Immunomodulatory
activity of Sulforaphane: A naturally
occurring isothiocyanate from broccoli
(Brassica oleracea). Phytomedicine 2007;14:
–545.
Geetha S, Singh V, Ram MS, Ilavazhagan G,
Banerjee PK, Sawhney RC.
Immunomodulatory effects of Seabuckthorn
(Hippophaea rhamnoides L.) against
chromium (VI) induced immunosuppression.
Mol. Cell. Biochem 2005;278:101–109.
Devasagayam TPA, Sainis KB. Immune
system and antioxidants: especially those
derived from Indian medicinal plants. Ind. J.
Exp. Biol 2002;40:639–655.
Deharo E, Baelmans R, Gimenez A, Quenevo
C, Bourdy G. In vitro immunomodulatory
activity of plants used by the Tecana ethnic
group in Bolivia. Phytomedicine
;11:516–522.
Sonel M, Kuttan G. Immunomodulatory and
antitumour activities of Tinospora cordifolia.
Fitoterapia 1999;70: 35–43.
Davis L, Kuttan G. Immunomodulatory
activity of Withania somnifera. J.
Ethnopharmacol 2000;71:193–202.
Sunila ES, Kuttan G. Immunomodulatory and
antitumour activity of Piper longum Linn and
Piperine. J. Ethnopharmacol 2004;90:339–
Haijto T, Hostanska K, Gabius HJ.
Modulatory potency of the B-galactoside
specific lectin from mistletoe extract (Iscador)
on the host defense system in vivo in rabbits
and patients. J. Cancer Res.1989;49: 4803–
uttan G, Kuttan R. Immunomodulatory
activity of a peptide isolated from Viscum
album extract. Immunol. Invest
;21:285–296.
Hookey HL, Van Ettan CH, Daxenbichler
ME. Glucosinolates. In: Liener, I.E. (Ed.),
Toxic Constituents of Plant Stuffs. Acaden
press, New York, p. 1980;103–142.
M Rinku, V Prasanth, Parthasarathy G.
Immunomodulatory activity of the methanolic
extract of Urena lobata Linn.The Internet
Journal of Pharmacology 2009;7:(1).
Khajuria Anamika, Gupta Amit, Garai
Saraswati, Wakhloob Purinima Basanti.
Immunomodulatory effects of two sapogenins
and 2 isolated from Luffa cylindrica in
Balb/C mice. Bioorganic & Medicinal
Chemistry Letters 2007;17:1608–1612.
Naznin Ara, Mamunur Rashid, Md Shah
Amran. Comparison of Moringa oleifera
Leaves Extract with Atenolol on Serum
triglyceride, Serum Cholesterol, Blood
glucose, heart weight, body weight in
Adrenaline Induced Rats. Saudi Journal of
Biological Sciences 2008
December;15(2):253-258.
Sunilson JAJ, P Jayaraj, Mohan MS, Kumari
Gnana AA, Varatharajan R. Antioxidant and
hepatoprotective effect of the roots of
Hibiscus esculentus Linn. International
Journal of Pharmacy 2009 July- Sep;3(3).
Shah NB, Nayak BS, Bhatt SP, Jalalpure SS,
Seth AK. The Anti-Inflammatory Activity Of
The Leaves Of Colocasia Esculenta. Saudi
Pharmaceutical Journal 2007July-Oct;15:3-4,
Micha Zimecki, Zbigniew Wieczorek.
Differential Patterns Of Cyclosporine A
Induced Inhibition Of Humoral And Cellular
Immune Responses To Sheep Erythrocytes In
Mice, pollish journal of pharmacology, 2001,
, 495-500.
Anwei Cheng, Fachun Wan, Jiaqi Wang,
Zhengyu Jin, Xueming Xu. Macrophage
immunomodulatory activity of
polysaccharides isolated from Glycyrrhiza
uralensis fish, International
Immunopharmacology 2008;8:43–50.
Kumar CSS, Malla TM, Ujjwala Kulkarni,
Sameena Akhter, N Ganesh. Amido black 10
B is preferable to Ponceau S stain for staining
Serum proteins in Electrophoretic studies
forclinical diagnosis. Intrnational Journal of
Physical Scienceses 2009;21(1).
Friedman S H. A standardized procedure for
serum protein electrophoresis on cellulose
acetate membrane strips. Clinica Chimica
Acta 1961;6(6);775-781