Coumarin isolation and comparative study of biological activities of Pterocaulon alopecuroides DC and Pterocaulon lorentzii Malme
Keywords:
Pterocaulon, Asteraceae, Phytotherapy, Antioxidant, ToxicityAbstract
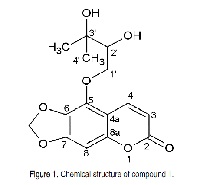
5-(2,3-Dihidroxy-3-methylbuthyloxy)-6,7-methylenedioxycoumarin was isolated from the chloroform extract of the two Asteraceae species Pterocaulon alopecuroides DC. and Pterocaulon lorentzii Malme. The structure was elucidated through IR and 1H and 13C NMR analyses. The extracts and the isolated compound did not exhibit toxic activity, as determined through the brine shrimp lethality method, and did not interfere with the integrity of erythrocytes, as demonstrated through a hemolytic assay. The antioxidant activities were investigated through three methods. In the phosphomolybdenum test, the ethyl acetate fraction of P. alopecuroides exhibited an antioxidant activity of 137.7% compared with rutin (positive control), and the ethyl acetate fraction of P. lorentzii exhibited an antioxidant activity of 101.7% compared with vitamin C (positive control). The two ethyl acetate fraction also exhibited excellent activity through the DPPH assay: P. alopecuroides and P. lorentzii exhibited IC50 values of 10.74 μg/ml and 7.63 μg/ml, respectively. In the TBARS bioassay, the crude extracts showed the more significant results: IA% 0.419 ± 0.0517 for P. alopecuroides and IA% 0.213 ± 0.0094 for P. lorentzii.
References
Stein AC, Alvares S, Avancini C, Zacchino S, Poser, GLV. Antifungal activity of some coumarins obtained from species of Pterocaulon (Asteraceae). Journal of Ethnopharmacology., 2006: 95-98. DOI: http://dx.doi.org/10.1016/j.jep.2006.02.009
Lima LFP, Matzenbacher NI. O gênero Pterocaulon Ell. (Asteraceae – plucheeae) no estad o Rio Grande do Sul, Brasil. Iheringia, Sér. Bot., 2008; 63 (2): 213-229. URL: http://www.fzb.rs.gov.br/publicacoes/iheringia-botanica/Ih63-2-p213-230.pdf
Daboit TC, Stopiglia CDO, Von Poser GL, Scroferneker ML. Antifungal activity of Pterocaulon alopecuroides (Asteraceae) against chromoblastomycosis agents. Mycoses, 2009; 53: 246-250. DOI: 10.1111/j.1439-0507.2009.01704.x
Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, Mclaughlin JL. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982; 45: 31-34. URL: https://www.thieme-connect.com/DOI/DOI?10.1055/s-2007-971236.
OMS, Quality Control Methods For Medicinal Plant Material. 1998. p. 41.
Flach J, Karnopp C, Corção G. Biofilmes formados em matéria-prima em contato com leite: fatores de virulência envolvidos. Acta Sci Vet. 2005; 33 (3): 291-296.
Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenium complex: specific application to the determination of vitamina E. Anal Biochem. 1999; 269: 337-341. URL: http://dx.doi.org/10.1006/abio.1999.4019.
Sousa CMM, Silva HR, Vieira-Jr GM, Ayres MCC, Costa CLS, Araújo DS, Cavalcante LCD, Baroos EDS, Araújo PBM, Brandão MS, Chaves MH. Fenóis totais e atividade antioxidante de cinco plantas medicinais. Química Nova. 2007; 30: 351-355. URL: http://dx.doi.org/10.1590/S0100-40422007000200021
Morais SM, Catunda Junior FEA, Silva, ARA, Martins Neto JS. Atividade antioxidante de óleos essenciais de espécies de Croton do nordeste do Brasil. Quim. Nova. 2006; 29(5): 907-910. URL: http://dx.doi.org/10.1590/S0100-40422006000500004
Barbosa LCA, Espectroscopia no infravermelho na caracterização de compostos orgânicos.Viçosa: Ed. UFV, 2007:48-53.
Debenedetti SL, De Kimpe N, Boeykens M, Coussio JD, Kesteleyn B. Structural revision of four coumarins from pterocaulon species. Phytochemistry. 1997; 45: 1515-1517.
Heemann ACW, Miguel OG, Miguel MD, Sasaki CM, Monache FD. Estudo fitoquímico da espécie Pterocaulon interruptum. RBCF. Revista Brasileira de Ciências Farmacêuticas. 2006; 42: 585-588. URL: http://www.scielo.br/pdf/rbcf/v42n4/a14v42n4.pdf