Lipid peroxidation inhibition by ethanolic extract and fractions from Rhamnus sphaerosperma var. pubescens (Reissek) M.C. Johnst. (Rhamnaceae)
Keywords:
Rhamnus sphaerosperma var. pubescens, lipid peroxidation, antioxidant, ethanolic extract, stigmasterol and sitosterolAbstract
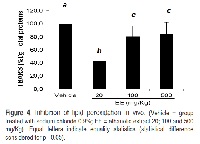
Rhamnus sphaerosperma var. pubescens is a species native to Brazil, found in the southern region, and still there is no data in the literature about biological activities of this plant. Therefore, the aims of this study were to verify the antioxidant activity and determine the ability to inhibit lipid peroxidation of crude ethanolic extract and it fractions hexane, chloroform and ethyl acetate of the stem. The total antioxidant activity was evaluated by phosphomolybdenum complex method. And the ability to inhibit lipid peroxidation was evaluated, in vitro and in vivo experiments, by quantification of thiobarbituric acid reactive species formed from peroxidation induced. The in vitro test about lipoperoxidation, were used lipids obtained from egg yolk. And, for in vivo assay, the peroxidation was determined only for crude extract, using stomachs of female rats injured by administration of absolute ethanol. The samples of Rhamnus sphaerosperma var. pubescens demonstrated an important antioxidant capacity. In the phosphomolybdenum complex method, the chloroform fraction (86.4%) and ethyl acetate fraction (96.2%) showed the best activity than crude extract. For the in vitro induced lipid peroxidation, the chloroform (63.3%) and hexane (59.7%) fractions demonstrated high capacity in prevention of lipid oxidation. The crude extract showed effectiveness in both methods, and was used in the verification of inhibition of lipid peroxidation in vivo. In this test, the lesser dose tested, 20 mg/Kg, showed better effectiveness in inhibiting lipid oxidation, with reduction of 56.9%. Furthermore, was possible identify the stigmasterol and sitosterol as compounds of this plant. Rhamnus sphaerosperma var. pubescens stem possess potential antioxidant activity, reducing lipid peroxidation tested by in vitro and in vivo methods.
References
. Schafer FQ, Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med. 2001; 30(11):1191-212. URL: http://dx.doi.org/10.1016/S0891-5849(01)00480-4.
. Niki, E. Assessment of antioxidant capacity in vitro and in vivo. Free Radic Biol Med. 2010; 49(4):503-515. URL: http://dx.doi.org/10.1016/j.freeradbiomed.2010.04.016.
. Ammar RB, Sghaier MB, Boubaker J, Bhouri W, Naffeti A, Skandrani I, Bouhlel I, Kilani S, Ghedira K, Chekir-Ghedira L. Antioxidant activity and inhibition of aflatoxin B1-, nifuroxazide-, and sodium azide-induced mutagenicity by extracts from Rhamnus alaternus L. Chem Biol Interact. 2008;174(1):1-10. URL: http://dx.doi.org/10.1016/j.cbi.2008.04.006.
Ammar RB, Neffati A, Skandrani I, Sghaier MB, Bhouri W, Ghedira K, Chekir-Ghedira L. Anti-lipid peroxidation and induction of apoptosis in the erythroleukaemic cell line K562 by extracts from (Tunisian) Rhamnus alaternus L. (Rhamnaceae). Nat Prod Res. 2011; 25(11):1047-58.
. Ng LT, Lin CC, Lu CM. Antioxidative effects of 6-Methoxysorigenin and Its Derivatives from Rhamnus nakaharai. Chem Pharm Bull. 2007; 55(3):382-4. URL: http://dx.doi.org/10.1248/cpb.55.382.
. Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999; 269(2):337-41. URL: http://dx.doi.org/10.1006/abio.1999.4019.
. Kalegari M, Miguel MD, Philippsen AF, Dias JFG, Zanin SMW, Lima CP, Miguel OG. Antibacterial, allelopathic and antioxidant activity of extracts and compounds from Rourea induta Planch. (Connaraceae). Journal of Applied Pharmaceutical Science. 2012; 2 (09):061-066.
. Morais SM, Catunda-Junior FEA, Silva ARA, Martins-Neto JS, Rondina D, Cardoso JHL. Antioxidant activity of essential oils from Northeastern Brazilian Croton species. Quim Nova. 2006; 29(05):907-910.
. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979; 95(2):351-8.
. Maróstica-Junior MR, Silva TAAR, Franchi GC, Nowill A, Pastore GM, Hyslop S. Antioxidant potential of aroma compounds obtained by limonene biotransformation of orange essential oil. Food Chemistry. 2009; 116:8–12.
. Kongduang D, Wungsintaweekul J, De-Eknamkul W. Biosynthesis of b-sitosterol and stigmasterol proceeds exclusively via the mevalonate pathway in cell suspension
cultures of Croton stellatopilosus. Tetrahedron Lett. 2008; 49(18):4067–4072. URL: http://dx.doi.org/10.1016/j.tetlet.2008.04.049.
. Kamalakkannan N, Prince PS. Antihyperglycaemic and antioxidant effect of rutin, a polyphenolic flavonoid, in streptozotocin-induced diabetic wistar rats. Basic Clin Pharmacol Toxicol. 2006; 98(1):97-103.
. Wei BL, Lu CM, Tsao LT, Wang JP, Lin CN. In Vitro Anti-Inflammatory Effects of Quercetin 3-O-Methyl Ether and Other Constituents from Rhamnus Species. Planta Med. 2001; 67(8):745-7.
. Liu J, Yeo HC, Doniger SJ, Ames BN. Assay of aldehydes from lipid peroxidation: gas chromatography-mass spectrometry compared with thiobarbituric acid. Anal Biochem. 1997; 245(2):161-6. URL: http://dx.doi.org/10.1006/abio.1996.9990.
. Pan JS, He SZ, Xu HZ, Zhan XJ, Yang XN, Xiao HM, Shi HX, Ren JL. Oxidative stress disturbs energy metabolism of mitochondria in ethanol-induced gastric mucosa injury. World J Gastroenterol. 2008; 14(38):5857-67. URL: http://www.wjgnet.com