Study of antioxidant and free radical scavenging activities of methanolic extract of Rumex acetosella roots and its fractions in different solvents
Keywords:
Rumex acetosella roots, antioxidants, free radical scavenging, flavonoidsAbstract
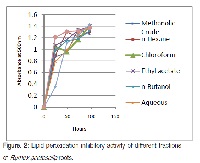
The context and purpose of the study: In view of the wide ethnomedicinal applications of Rumex acetosella, and as part of our quest for natural antioxidants, the present research was designed to evaluate antioxidant and free radical scavenging activities of the methanolic extract of its roots and its sub-fractions in solvents of different polarity employing DPPH free radical scavenging, ABTS, FRAP, phosphomolybdate, reducing power and lipid peroxidation assays. Main findings: The MeOH extract and its fractions exhibited considerable antioxidant potential. The n-butanol fraction, having highest phenolic and flavonoid contents, 252.19 μg/mL of gallic acid equivalent and 891.34 μg/mL of rutin equivalent respectively, was most potent. All the fractions efficiently scavenged the DPPH free radical; n-butanol fraction was most powerful and had the lowest EC50 (212.36 μg/ mL) and TEC50 (4 min). The phosphomolybdate antioxidant activity of the plant extracts ranged from 325.41-82.47 μg/mL of AAE (Ascorbic Acid Equivalent). The n-butanol fraction had the highest FRAP, or ferric reducing antioxidant potential, value (569.52 μg/mL of AAE) and the highest Trolox equivalent antioxidant capacity, or TEAC, value (1747.71 mM) in ABTS assay. The chloroform fraction that was least active in all the assays showed the lowest TEAC value (638.87 mM). Brief summary and potential implications: The polar fractions of the MeOH extract of the roots of R. acetosella, having higher phenolics and flavonoids, displayed noteworthy antioxidant properties, the n-butanol fraction being the most powerful. Results present the plant as a potential source of natural antioxidants.
References
Huang D, Ou B, Prior RL. The chemistry behind antioxidant assays. J Agric Food Chem. 2005; 53: 1841-1856.
Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci. 2008; 4(2): 89-96.
Kumar S. Free radicals and antioxidants: Human and food system. Adv Appl Sci Res. 2011; 2(1): 129-135.
Young IS, Woodside JV. Antioxidants in health and disease. J.Clin Pathol. 2001; 54: 176-186.
Flora SJS. Structural, chemical and biological aspects of antioxidants for strategies against metal and metalloid exposure. Oxid Med Cell Longev. 2009; 2(4): 191-206.
Branen AL. Toxicology and biochemistry of butylated hydroxyanisole and butylated hydroxytoluene. Ameri Oil Chemists Soci. 1975; 5: 59-63.
Barlow SM. Toxicological Aspects of Antioxidants Used as Food Additives, in Food Antioxidants. Hudson BJF, editor. Amsterdam: Elsevier; 1990. p. 23.
Djeridane A, Yousfi M, Nadjemi, B, Boutassouna D, Stocker P, Vidal. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. N. Food Chem. 2006; 97: 654-660.
Ayoola GA, Sofidiya T, Odukoya O, Coker HAB, Phytochemical screening and free radical scavenging activity of some Nigerian medicinal plants. J Pharm Sci Pharm Pract. 2006; 89(3,4): 133-136.
Lako J, Trenerry VC, Wahlqvist M, Wattanapenpaiboon N, Sotheeswaran S, Premier R. Phytochemical flavonols, carotenoids and the antioxidant properties of a wide selection of Fijian fruit, vegetables and other readily available foods. Food Chem. 2007; 101: 1727-1741.
Hinneburg I, Dorman HJD, Hiltunen R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006; 97: 122-129.
Ahmed D, Arshad MA, Asghar MN, Ikram M, Antioxidant and Free Radical Scavenging Potential of Otostegia limbata. Asian J Chem. 2010; 22 (6): 4524-32.
Ruiz-Terán F, Medrano-Martínez A, Navarro-Ocaña A. Antioxidant and Free radical scavenging activities of plant extracts used in traditional medicine in Mexico. Afri J Biotechnol. 2008; 7(12): 1886-1893.
Ali SI, Qaiser M. A phytogeographical analysis of the phanerogames of Pakistan and Kashmir. Proc Royal Soc Edinburg. 1986; 89B: 89-101.
Elzaawely AA, Tawata S. Antioxidant capacity and phenolic content of Rumex dentatus L. grown in Egypt. J Crop Sci Biotechnol. 15(1): 59-64.
Yuan Y, Chen WS, Zheng SQ, Yang GJ, Zhang WD, Zhang HM. Studies on chemical constituents in root of Rumex patientia L. Zhongguo Zhong Yao Za Zhi. 2001; 26(4): 256-258.
Shuying G, Bo F, Ruonan Z, Jiankang M, Wei W. Preparative isolation of three anthraquinones from Rumex japonicus by high-speed counter-current chromatography. Molecules. 2011; 16(2): 1201-1210.
Abd el-Fattah H, Gohar A, el-Dahmy S, Hubaishi A. Phytochemical investigation of Rumex luminiastrum. Acta Pharm Hung. 1994; 64(3): 83-5.
Mei R, Liang H, Wang J, Zeng L, Lu Q, Cheng Y. New seco-anthraquinone glucosides from Rumex nepalensis. Planta Med. 2009; 75(10): 1162-1164.
Fan JP, Zhang ZL. Studies on the chemical constituents of Rumex crispus. Zhong Yao Cai. 2009; 32(12): 1836-40.
Tavares L, Carrilho D, Tyagi M, Barata D, Serra AT, Duarte CM, Duarte RO, Feliciano RP, Bronze MR, Chicau P, Espírito-Santo MD, Ferreira RB, dos Santos CN. Antioxidant capacity of Macaronesian traditional medicinal plants. Molecules. 2010; 15(4): 2576-2592.
Baig H, Ahmed D, Zara S, Aujla MI, Asghar MN. In vitro Evaluation of Antioxidant Properties of Different Solvent Extracts of Rumex acetosella Leaves. Oriental J Chem. 2011; 27(4); 1509-1516.
Singleton VL, Rossi JR Jr. Colorimetry of total phenolics with phosphomolybdic-Phosphotungstic acid. Am J Enol Vitic. 1965; 16:144-148.
Slinkard K, Singleton VL. Total phenol analysis automation and comparison with manual methods. Am J Enol Vitic. 1977; 28: 49-55.
Ahmed D, Zara S, Baig H. In vitro Analysis of Antioxidant Activities of Oxalis corniculata Linn. Fractions in Various Solvents. Afri J Altern Compl Med. 2013; 10(1): 158-165.
Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm.-Wiss.u.-Technol. 1995; 28: 25-30.
Umamaheswari M, Chatterjee TK. In vitro antioxidant activities of the fractions of Cocinnia grandis. Afri J Trad Compl Altern Med. 2008; 5(1), 61-73.
Benzie FF, Strain J. Ferric reducing / Antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power. J Methods Enzymol. 1999; 299: 15-23.
Oyaizu M. Studies on products of browning reactions: antioxidative activities of products of browning reaction prepared from glucosamine. Japanese J Nutrition. 1986; 44: 144-158.
Re R, Pellegrini N, Proteggente A, Pannala AM, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radical Biol Med. 1999; 26: 1231-1237.
Mitsuda H, Yasumoto K, Iwami K. Antioxidative action of indole compounds during the autooxidation of linoleic acid. Eiyoto Shokuryo. 1996; 19, 210-214.
Miliauskas G, Venskutonis PR, Van-Beck TA. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004; 85: 231-237.
Rasool N, Rızwan K, Zubaır M, Kaleem Ur Rahman Naveed KUR, Imran I and Ahmed VU, Antioxidant potential of different extracts and fractions of Catharanthus roseus shoots. Int J Phytomed. 2011; 108-114.
Ju-Sung Kim, Myong-Jo Kim. In vitro antioxidant activity of Lespedeza cuneata methanolic extracts. J Medi Plants Res. 2010; 4(8): 674-679.
Foti MC, Daquino C, Geraci C. Electron-transfer reaction of cinnamic acids and their methyl esters with the DPPH radical in alcoholic solutions. J Org Chem. 2004, 69, 2309-2314.
Kai W, Yingming P, Hengshan W, Ye Z, Qian L, Zhiren Z, Haiyun L, Min L. Antioxidant activities of Liquidambar formosana Hance leaf extracts. Med Chem Res. 2010; 19(2): 166-176.
Alam, MB, M.Sarowar Hossain, Nargis Sultana Chowdhury, M. Ehsanul Haque Mazumder, M. Ekarmul Haque, Anwarul Islam. In vitro and in vivo Antioxidant and Toxicity Evaluation of Different Fractions of Oxalis corniculata Linn. J Pharmaco Tox. 2011; 6(4): 337-348.
Rahman A., Rahman, M. M., Sheik, M. M. I., Rahman, M. M., Shadli, S. M. and Alam, M. F. Free Radical scavenging activity and phenolic content of Cassia sophera L. Afr J Biotechnol. 2008; 7: 1591-1593.
Shinde AN, Malpathak N, Fulzele DP. Dtermination of isoflavne content and antioxidant activity in Psoralea coryifolia L. callus cultures. Food Chem. 2010; 118: 128–132.
Bors W, Heller W, Michel C, Saran M. Flavonoids as antioxidants: Determination of radical scavenging efficiencies. Methods Enzymol. 1990; 186: 343-355.
Nabavi SM, Ebrahimzadeh MA, Nabavi SF, Fazelian M, Eslami B. In vitro antioxidant and free radical scavenging activity of Diospyros lotus and Pyrus boissieriana growing in Iran. Pharmacognosy Magazine. 2009; 4(18): 123-127.
Heim KE, Tagliaferro AR., Bobilya DJ. Flavonoid antioxidants: Chemistry, metabolism and structure- activity relationships. J Nutr Biochem. 2002; 13: 572-584.
Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complem Altern Med. 2012; 12: 221.