Identification of β-carotene and β-sitosterol in methanolic extract of Dipteracanthus patulus (Jacq) nees and their role in antimicrobial and antioxidant activity
Keywords:
Dipteracanthus patulus, HPTLC, β-carotene, β-sitosterol, iridoid glycosides, Antioxidant activity, Antimicrobial activityAbstract
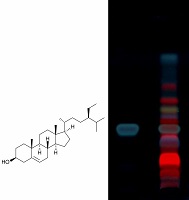
The Dipteracanthus patulus (Jacq) nees is undershrub belonging to the family acantheaceae. Antimicrobial activity (disc diffusion method) and antioxidant activity by different in-vitro methods (DPPH, hydrogen peroxide, nitric oxide radical scavenging and reducing power) of methanolic extract of Dipteracanthus patulus (MEDP) was evaluated. The qualitative and quantitative estimation of β-carotene and β- sitosterol in MEDP was carried out by high performance thin layer chromatography (HPTLC). The total phenolic content of was determined by Folin-Ciocalteu method. Experimental findings indicate promising antimicrobial activity (antibacterial and antifungal) and potent antioxidant activity of MEDP. In addition, phytochemical analysis and spectral studies of MEDP were also performed. It is presumed that antimicrobial and antioxidant activity observed with MEDP may largely be attributed to the presence of major phytoconstituents (β-carotene, β-sitosterol and iridoid glycosides) and other minor components may participate as promoters.
References
Chirangini P, Amit K, Pandey BN,
Sharma GJ, Mishra KP. Herbals for
human health and disease: prospects and
challenges in 21st century. In: Sharma RK,
Arora R, editors. Herbal Drugs - A
Twenty First Century Perspective. New
Delhi: Jaypee Brothers Medical
Publishers; 2006;p. 1-7.
Piddock LJV, Wise R. Mechanisms of
resistance to quinolones and clinical
perspectives. J. Antimicro. Chemother.
;23(4),475-480.
Parida MM, Dash PK, Saxena P, Jana
AM, Rao PVL. Perspectives on
antimicrobial activity of natural plant
products. In: Sharma RK, Arora R,
editors. Herbal Drugs - A Twenty First
Century Perspective. New Delhi: Jaypee
Brothers Medical Publishers; 2006;p.
-496.
Narasimhan S, Shobana R, Sathya TN.
Antioxidants- natural rejuvenators that
heal, detoxify and provide nourishment.
In: Sharma RK, Arora R, editors. Herbal
Drugs - A Twenty First Century
Perspective. New Delhi: Jaypee Brothers
Medical Publishers; 2006;p.548-558.
Chopra RN, Nayar SL, Chopra IC.
Glossary of Indian Medicinal Plants. New
Delhi: Council of Scientific and Industrial
Research. 1986;p.99.
Akthar MF. Chemical and biological
investigations of medicinal herbs phyla
nodiflora, ruellia patula and ruellia
brittoniana. Dissertation submitted to
University of Karachi, Department of
Pharmacognosy, Faculty of Pharmacy,
Pakistan. 1993;p. 59.
Manikandan A, Doss DVA. Evaluation of
biochemical contents, nutritional value,
trace elements, SDS-PAGE and HPTLC
profiling in the leaves of Ruellia tuberosa
L. and Dipteracanthus patulus (Jacq.). J.
Chem. Pharm. Res. 2010;2(3):295-303.
Saroja K, Elizabeth JD, Gopalkrishnan S.
Wound healing activity of leaves of
Dipteracanthus patulus (Jacq.).
Pharmacologyonline. 2009;2:462-469.
Manikandan A, Doss DVA. Effect of 50%
hydroethanolic leaf extracts of Ruellia
tuberosa L. and Dipteracanthus patulus
(Jacq.) on AST, ALT, ACP and ALP
levels in serum, liver and kidney of
alloxan induced diabetic rats. Anna.
Pharm. Pharm. Sci. 2010;1(2):142-146.
Akhtar MF, Rashid S, Ahmad M,
Usmanghani K. Cardiovascular evaluation
of Ruellia patula and Ruellia brittoniana.
J. Islamic. Acad. Sci. 1992;5(1):67-71.
Ansari SH. Essentials of Pharmacognosy.
Delhi: Birla Publication; 2006;p.357-383.
Harborne JB. Phytochemical methods: A
guide to modern techniques of plant
analysis. London: Chapman and Hall
Publication; 1998;p. 119.
Slinkard K, Singleton VL. Total phenol
analysis: automation and comparison with
manual methods. Am. J. Enol. Viticult.
;28:49–55.
Chen Y, Wang MF, Rosen RT, Ho CT. 2,
-Diphenyl -1 -picrylhydrazyl radicalscavenging active components from
Polygonum multiflorum Thunb. J. Agric.
Food Chem. 1999;47:2226–2228.
Green LC, Wagner DA, Glogowiski J,
Skipper PL, Wishnok JS, Tannenbaum
SR. Analysis of nitrate, nitrite and 15N
nitrate in biological fluids. Anal.
Biochem. 1982;126:131-138.
Ruch RJ, Cheng SJ, Klaunig JE.
Prevention of cytotoxicity and inhibition
of intercellular communication by
antioxidant catechins isolated from
Chinese green tea. Carcinogenesis.
;10:1003–1008.
Oyaizu M. Studies on products of
browning reactions: antioxidative
activities of products of browning reaction
prepared from glucosamine. Jpn. J. Nutr.
;44:307–315.
Benzie IFF, Strain JJ. The ferric reducing
ability of plasma (FRAP) as a measure of
antioxidant power: The FRAP assay.
Anal. Biochem. 1996;239:70–76.
Wong SP, Leong LP, Koh JHW.
Antioxidant activities of aqueous extracts
of selected plants. Food Chem.
;99:775-783.
Kirby MDK, Bauer RW, Sherris JC,
Turck M. Antibiotic susceptibility testing
by standard single disc diffusion method.
Am. J. Clin. Pathol. 1966;45:493-496.
Godinho A, Bhosale S. Carotenes
produced by alkaliphilic orangepigmented strain of microbacterium
arborescens-AGSB isolated from coastal
sand dunes. Indian J. Mar. Sci.
;37(3):307-312.
Yamaguchi T, Takamura H, Matoba T,
Terao J. HPLC method for evaluation of
the free radical-scavenging activity of
foods by using 1,1,-diphenyl-2-
picrylhydrazyl. Biosci. Biotech. Biochem.
;62:1201–1204.
Chen CW, Ho CT. Antioxidant properties
of polyphenols extracted from green and
black tea. J. Food Lipids. 1995;2:35–46.
Bors W, Heller W, Michael C, Saran M.
Radical chemistry of flavonoids
antioxidants. Adv. Exp. Med. Biol.
;264:165–170.
Djeridane A, Yousfi M, Nadjemi B,
Boutassouna D, Stocker P, Vidal N.
Antioxidant activity of some Algerian
medicinal plants extracts containing
phenolic compounds. Food Chem.
;97:654–660.
Senthilkumar N, Badami S, Dongre SH,
Bhojraj S. Antioxidant and
hepatoprotective activity of methanolic
extract of Careya arborea bark in Ehrlich
ascites carcinoma bearing mice. J. Nat.
Remedies. 2008;62:336-339.
Da Silva VS, Silvab GH, Bolzani VDS,
Lopes MN. Isolation of lignin glycoside
from Alibertia sessilis (Vell) K. Schum
(Rubiaceae) by preparative HPLC.
Ecletica Quimica 2006;31(4):55-58.
Sies H, Stahl W. Vitamin E and C, beta
carotene and other carotenoids as
antioxidants. Am. J. Clin. Nutr.
;62:15S-21S.
Scalbert A. Antimicrobial properties of
tannins. Phytochemistry. 1991;30:3875-
Hagerman AE, Butler LG. The specificity
of proanthocyanidin-protein interactions.
The J. Biol. Chem. 1981;256:4494–4497.
Haslam E. Natural polyphenols (vegetable
tannins) as drugs: possible modes of
action. J. Nat. Prod. 1996;59:205–215.
Beltrame FL, Pessini GL, Doro DL, Filho
BPD, Bazotte RB, Cortez DAG.
Evaluation of the antidiabetic and
antibacterial activity of Cissus sicyoides.
Braz. Arch. Biol. Tech. 2002;45(1):21-
Akunyili DN, Houghton PJ, Raman A.
Antimicrobial activities of the stembark of
Kigelia pinnata. J. Ethonopharmacol.
;35(2):173-177.
Lee DG, Jung HJ, Woo ER. Antimicrobial
property of (+)-lyoniresinol-3alpha-Obeta-D-glucopyranoside isolated from the
root bark of Lycium chinense Miller
against human pathogenic
microorganisms. Arch. Pharm. Res.
;28(9):1031-1036.