Antibacterial, Antioxidant activity and Phytochemical studies of Crossandra infundibuliformis leaf extracts
Keywords:
Crossandra infundibuliformis, leaf extracts, antibacterial activity, phytochemicals, antioxidant activityAbstract
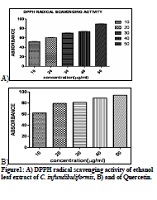
The aim of the present study was to evaluate Crossandra infundibuliformis (Acanthaceae family) for its antibacterial, antioxidant activity and phytochemical constituents. The leaves of C. infundibuliformis were screened for antibacterial activity against six pathogenic bacteria isolated from clinical samples such as Shigella dysenteria, Pseudomonas aeruginosa, Serratia marcescens, Salmonella typhimurium, Proteus mirabilis & Staphylococcus aureus by extracting them in ethanol, petroleum ether and water. Ethanol extract exhibited inhibition zone against all the pathogens comparable to the standard (Amikacin), whereas no zone of inhibition was observed in petroleum ether and water extracts. Phytochemical analysis showed the presence of flavonoids, saponins, terpenoids, cardiac glycoside, reducing sugars and tannins. The ethanol extract of C. infundibuliformis leaves appeared to be good antioxidant agent with maximum inhibition percentage of 89.27 ±0.284 % at 16µg/ml whereas standard Quercetin showed maximum inhibition percentage of 94.30 ±0.272 % at 22µg/ml.
References
Shanmugam S, Gayathiri N, Sakthivel B,
Ramar S, Rajendran K. Plants used as
medicine by Paliyar tribes of
Shenbagathope in Virudhunagar district
of Tamil Nadu, India. Ethanobotanical
leaflets. 2009;13:370-378.
Kumar SA, Sumalatha K, Lakshmi S.
Aphrodisiac Activity of Crossandra
infundibuliformis (L.) on Ethanol induced
Testicular Toxicity in male rats.
Pharmacologyonline. 2010;2:812-817.
Madumita G, Saral AM, Senthilkumar B,
Sivaraj A. Hepatoprotective potential of
petroleum ether leaf extract of
Crossandra infundibuliformis on CCl4
induced liver toxicity in albino mice.
Asian Pacific Journal of Tropical
Medicine. 2010;3:788-790.
Priya SV, Saratha R. Crossandra
infundibuliformis leaves as an effective
inhibition for mild steel corrosive in 1M
HCl [Abstract]. Ajrc, 2010;3:434.
Ayoola GA, Coker HAB, Adesegun SA,
Adepoju-Bello AA, Obaweya K, Ezennia
EC, Atangbaljila TO. Phytochemical
screening and Antioxidant activities of
some selected medicinal plants used for
malaria therapy in Southwestern Nigeria.
Tropical J of Pharmaceutical Research.
;7(3):1019-1024.
Blois MS. Antioxidant determinations by
the use of a stable free radical. Nature.
;181:1199-1200.
Nmorsi OPG, Ukwandu NCD,
Egwunyenga AO. Antioxidant status of
Nigerian children with Plasmodium
falciparum malaria. African J of
Microbiology Research. 2007;5:61-64.
Tsuchiya H, Sato M, Miyazaki T,
Fujuwara S, Tanigaki S, Ohayama M,
Tanaka T, Linuma M. Comparative study
on the antibacterial activities of
phytochemical flavonones against
methicillin resistant Staphylococcus
aureus. J of Ethanopharmacology.
;50:27-34.
Ya C, Gaffney SH, Lilley TH, Haslam E.
Carbohydrate-Polyphenol Complexation.
In: Chemistry and Significane of
condensed tannins. Hemingway RW,
Karchesy JJ ed. In Plenum Press, New
York. 1988;553.
Nabila B, Mirice S, Puran A. Antioxidant
and Antimicrobial activities of Pistacia
lentiscus and Pistacia attantica extract.
Afri. J of Pharmacology. 2008;2:22-28.
Dewick PM. Medicinal Natural products:
A Biosynthetic approach. 2nd ed: England.
John Wiley and Sons ltd; 2002:p.241-243.
Rupasinghe HP, Jackson CJ, Poysa V,
Beriado CD, Bewley JD, Jenkinson J.
Soyasapogenol A and B distribution in
soybean (Glycine max L. Merr) in
relation to seed physiology, genetic
variability and growing location. J of
Agriculture and Food Chem.
;51:5888-5894.
Malairajan P, Gopalakrishnan G,
Narasimhan S, Veni KJK. Analgesic
activity of some Indian medicinal plants.
J Ethnopharmacol. 2006;106:425-8.
Argal A, Pathak AK. CNS activity of
Calotropis gigantea roots. J.
Ethanopharmacology. 2006;106.142-145.