Isolation of biologically active metabolites from Bougainvillea spectabilis Willd. cultivated in Egypt
Keywords:
anti-hepatotoxic, anti-oxidant, Bougainvillea spectabilis Willd, cytotoxic, flavonoidsAbstract
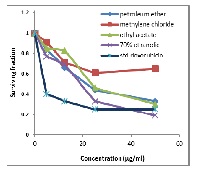
Bougainvillea spectabilis Willd. is an ornamental plant cultivated in tropical, subtropical regions and other places as Egypt. The present study aimed to perform bioassay guided fractionation and isolation of some of the bioactive compounds from the Egyptian cultivate. The total ethanol extracts of the leaves (T.ET.L.), stems (T.ET.S.) and flowers (T.ET.F.) were screened for some pharmacological activities viz. in vivo anti-oxidant and anti-hepatotoxic, in addition to in vitro cytotoxic activities. The anti-oxidant effect was assessed by measuring serum glutathione level (GSH) in alloxan-induced diabetic rats. The anti-hepatotoxic activity was evaluated via measuring serum markers level viz. alanine aminotransferase (ALT), aspartate aminotransferase (AST), and alkaline phosphatase (ALP) in CCl4-induced hepatotoxicity in rats. In vitro cytotoxicity of the different extracts was estimated for liver cancer cell line (HEPG2) adopting Sulforhodamine B stain assay. T.ET.L. exhibited significantly potent anti-oxidant and anti-hepatotoxic activities, while T.ET.S. showed the highest cytotoxic activity. Through biological guided fractionation, leaves and stems were subjected to successive solvent extraction, whereas the leaves ethyl acetate (Et.Ac.L.) and the stems ethanol 70% (Et.70%S.) extracts showed highly potent activities. Thus, different chromatographic techniques were performed on Et.Ac.L. and Et.70%S. extracts leading to the isolation of five bioactive metabolites. Three flavonoids were isolated from Et.Ac.L.; genistein-7-O-rutinoside (1), formononetin-7-O-rutinoside (2) and myricetin (3), while orobol-7-O-glucoside (4) and hesperidin (5) were isolated from Et.70%S. This work demonstrated the importance of the plant as a promising anti-oxidant, anti-hepatotoxic and cytotoxic product for nutraceutical use.
References
Singh KK. A review: Multiplication of Bougainvillea species through cutting. Int J Chem Stud. 2018; 6(2): 1961-1965. https://doi.org/10.20546/ijcmas.2017.602.132 [2] Farzana R, Nadia S, Ijaz A, Saima S, Fakher UN, Shagufta N. Phytochemical analysis and inhibitory activity of ornamental plant (Bougainvillea spectabilis) Asian J Plant Sci Res. 2013; 3(2): 1-5. [3] Srinivasan KK, Subramanian SS. Flavonoids of Bougainvillea spectabilis. Arogya. 1983; 9(2): 176-178. [4] Chang W, Lee Y, Lu F, Chiang H. Inhibitory effects of flavonoids on xanthine oxidase. Anticancer Res. 1993;13(6A): 2165-2170. [5] Chang WS, Chang YH, Lu FJ, Chiang H. Inhibitory effects of phenolics on xanthine oxidase. Anticancer Res. 1994; 14(2A): 501-506. [6] Narayanan CR, Joshi DD, Mujumdar AM, Dhekne VV. Pinitol a new antidiabetic compound from the leaves of Bougainvillea spectabilis. Curr Sci. 1987; 56(3): 139-141. [7] Sunil J, Yatendra K, Khan MSY. Isolation of antidiabetic principle from Bougainvillea spectabilis Willd. (Nyctaginacea) stem bark. Trop J Pharm Res. 2013; 12(5): 761-765. https://doi.org/10.4314/tjpr.v12i5.15 [8] Eid HH, El Deeb KS, Metwally GF, Abdel Halim MB. Influence of plant growth regulators on calluogenesis and secondary metabolites production in Bougainvillea spectabilis Willd. cultures. World J Pharm Res. 2015; 4(6): 296-313. [9] Balasaraswathi R, Sadasivam S, Chitra H, Raja J. Inhibition of in-vitro translation and cleavage of rRNA by Bougainvillea antiviral protein. Indian J Agric Biochem. 2001;14(1&2): 67-68. [10] Bhat MM, Kothiwale SK, Tirmale AR, Bhargava SY, Joshi BN. Anti-diabetic properties of Azardiracta indica and Bougainvillea spectabilis: in vivo studies in murine diabetic model. Evid Based Complement Alternat Med. 2011; 2011: 1-9. https://doi.org/10.1093/ecam/nep033 [11] Sandeep D, Mamta S, Sonam R, Meenakshi B, Manish K, Anil KC. Evaluation of antimicrobial and antioxidant activities of Bougainvillea spectabilis. Int J Pharm Pharm Sci. 2013; 5(3): 178-182. [12] Hembrom AR, Shail P, Singh VN. Anti-fertility effects of aqueous leaf extract of Bougainvillea spectabilis on seminal LDH isozymes in mice. J Appl Biosc. 2013;39(1): 60-62. [13] Saikia H, Lama A. The effect of Bougainvillea spectabilis leaves on serum lipids in albino rats with high fat diet. Int J Pharm Sci Drug Res. 2011; 3(2): 141-145. [14] Chauhan P, Mahajan S, Kulshrestha A, Shrivastava S, Sharma B, Goswamy HM, Prasad GB KS. Bougainvillea spectabilis exhibits antihyperglycemic and antioxidant activities in experimental diabetes. Evid Based Complement Alternat Med. 2016; 21(3): 177-185. https://doi.org/10.1177/2156587215595152 [15] Mandal G, Chatterjee C, Chatterjee M. Evaluation of anti-inflammatory activity of methanolic extract of leaves of Bougainvillea spectabilis in experimental animal models. Pharmacog Res. 2015; 7(1): 18-22. https://doi.org/10.4103/0974-8490.147194 [16] Mishra N, Tandonn VL, Dhama K, Khandia R, Munjal A. Does Bougainvillea spectabilis protect swiss albino mice from aflatoxin-induced hepatotoxicity? Adv Anim Vet Sci. 2016; 4(5): 250-257. https://doi.org/10.14737/journal.aavs/2016/4.5.250.257 [17] Do LT, Aree T, Siripong P, Vo NT, Nguyen TT, Nguyen PK, Tip-pyang S. Cytotoxic flavones from the stem bark of Bougainvillea spectabilis willd. Planta med. 2018; 84(02): 129-134. https://doi.org/10.1055/s-0043-118102 [18] Hasmida MN, Nur Syukriah AR, Liza MS, Mohd Azizi CY. Effect of different extraction techniques on total phenolic content and antioxidant activity of Quercus infectoria galls. Int Food Res J. 2014; 21(3): 1075-1079. [19] Agrawal M, Agrawal Y, Itankar P, Patil A, Vyas J, Kelkar A. Phytochemical and HPTLC studies of various extracts of Annona squamosa (Annonaceae). Int J Pharm Tech Res. 2012; 4(1): 364-368. [20] Karber G. Determination of LD50. Arch Exp Pathol Pharmacol. 1931; 162: 480. https://doi.org/10.1007/BF01863914 [21] Paget GE, Barnes JM. Toxicity tests. In: Laurence DR, Bacharach AL, editors. Evaluation of drug activities pharmacometries. Academic Press, London and New York; 1964. p.135. https://doi.org/10.1016/B978-1-4832-2845-7.50012-8 [22] Beutler E, Duron O, Kelly B. Improved Method for the Determination of Blood Glutathione. J Lab Clin Med. 1963; 61: 882-883. [23] Klassen CD, Plaa GL. Comparison of the biochemical alteration elicited in liver of rats treated with CCl4 and CHCl3. Toxic Appl Pharmacol. 1969; 18: 2019. [24] Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990; 82(13): 1107-1112. https://doi.org/10.1093/jnci/82.13.1107 [25] Lorke D. A new approach to practical acute toxicity testing. Arch Toxicol. 1983; 54(4): 275-287. https://doi.org/10.1007/BF01234480 [26] Marzouk MS, Ibrahim MT, El-Gindi OR, Abou Bakr MS. Isoflavonoid glycosides and rotenoids from Pongamia pinnata leaves. Z Naturforsch. 2008; 63(1-2): 1-7. https://doi.org/10.1515/znc-2008-1-201 [27] Mabry TJ, Markham KR, Thomas MB. The Scientific Identification of Flavonoids. 2nd ed. NewYork (USA): Springer Verlag; 1996. [28] He D, Gu D, Huang Y, Ayupbek A, Yang Y, Aisa HA, Ito Y. Separation and purification of phenolic acids and myricetin from black currant by high speed countercurrent chromatography. J Liq Chromatogr Relat Technol. 2009; 32(20): 3077-3088. https://doi.org/10.1080/10826070903320756 [29] Choi HJ, Bae EY, Song JH, Baek SH, Kwon DH. Inhibitory effects of orobol 7-O-D-glucoside from banaba (Lagerstroemia speciosa L.) on human rhinoviruses replication. Lett Appl Microbiol. 2010; 51(1): 1-5. https://doi.org/10.1111/j.1472-765X.2010.02845.x [30] Maltese F, Erkelens C, Kooy FV, Choi YH, Verpoorte R. Identification of natural epimeric flavanone glycosides by NMR spectroscopy. Food Chem. 2009; 116(2): 575-579. https://doi.org/10.1016/j.foodchem.2009.03.023 [31] Rice-Evans C, Miller N, Paganga G. Antioxidant properties of phenolic compounds. Trends plant sci. 1997; 2(4):152-159. https://doi.org/10.1016/S1360-1385(97)01018-2 [32] Ong KC, Khoo HE. Biological effects of myricetin. Gen. Pharmacol. 1997; 29(2): 121-126. https://doi.org/10.1016/S0306-3623(96)00421-1 [33] Mu H, Bai YH, Wang ST, Zhu ZM, Zhang YW. Research on anti-oxidant effects and estrogenic effect of formononetin from Trifolium protense red clover. Phytomedicine. 2009; 16(4): 314-319. https://doi.org/10.1016/j.phymed.2008.07.005 [34] Han RM, Tian YX, Liu Y, Chen CH, Ai XC, Zhang JP, Skibsted LH. Comparison of flavonoids and isoflavonoids as antioxidants. J Agric Food Chem. 2009; 57(9): 3780-3785. https://doi.org/10.1021/jf803850p [35] Nwaehujor CO, Ode JO, Nwinyi FC, Asuzu OV. Mechanism of action involved in the hepatoprotective activities of methanol extract of Cassytha filiformis L. aerial parts in CCl4-induced liver damage. Comp Clin Path. 2014; 23(6): 1749-1755. https://doi.org/10.1007/s00580-014-1997-4 [36] Al-Ashral HA, El-Sheltawy ST. Antioxidant capacity of hesperidin from Citrus peel using electron spin resonance and cytotoxic activity against human carcinoma cell lines. Pharm Biol. 2011; 49(3): 276-282. https://doi.org/10.3109/13880209.2010.509734 [37] De Stefani E, Boffetta P, Deneo-Pellegrini H, Mendilaharsu M, Carzoglio JC, Ronco A, Olivera L. Dietary antioxidants and lung cancer risk: a case-control study in Uruguay. Nutr Cancer. 1999; 34(1): 100-110. https://doi.org/10.1207/S15327914NC340114