In vitro Hepatoprotective potential of the whole plant of Fumaria indica (Haussk.) Pugsley and an isolated alkaloid Protopine
Keywords:
Fumaria indic, Ethanol extract, Protopine, Liver slice culture model, In vitro HepatoprotectiveAbstract
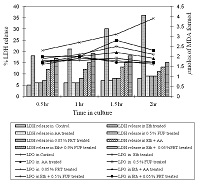
Fumaria indica (Haussk.) Pugsley, Fumariaceae [syn. F. vaillantii Loisel.] is an important medicinal plant known as ‘Fumitory’. Ethnobotanical and ayurvedic literature reports that the plant is used in treatment of liver diseases as well as diverse pharmacological activities. In present work, in vitro hepatoprotective effects of ethanol extract of F. indica (FUP) and alkaloid, protopine (PRT) on carbon tetrachloride induced oxidative stress has been demonstrated. An isoquinoline alkaloid, protopine was isolated from ethanol extract of F. indica and characterized by spectral data. Carbon tetrachloride (CCl4) and ethanol has been used as a hepatotoxin. Cytotoxicity was estimated by quantitating the release of lactate dehydrogenase (LDH) in culture medium along with antioxidant enzymes namely superoxide dismutase, catalase and glutathione reductase. HPLC profile of FUP and PRT was developed using water: methanol (7:3) as a mobile phase. CCl4 and ethanol induces 5.5 and 4 times more release of LDH from the liver cells and twice the amount of lipid peroxidation as compared to the cells from untreated liver tissue. These LDH and lipid perioxidation activities were reduced significantly in dose dependent manner after addition of FUP and PRT (at doses 0.5 % FUP and at does 0.025, 0.05 % PRT; p < 0.001). The activity of antioxidant enzymes was found to be elevated in CCl4/ethanol treated cells. However, after addition of FUP/PRT along with cytotoxicant the activities were lowered significantly. The peak of PRT has been detected in FUP at retention times 1.670. sBased on these studies it may be precluded that protopine from F. indica, as a possible therapeutic for preventing oxidative stress in vitro by boosting the antioxidant capacity of the liver.
References
Handa SS, Sharma A, Chakraborti KK. Natural products and plants as liver protecting drugs. Fitoterapia. 1986;57:307-351.
Handa SS, Sharma A, Chakraborti KK. Antihepatotoxic activity of some Indian herbal formulations as compared to Silymarin. Fitoterapia. 1991;62:229.
Satyavati GV, Raina MK, Sharma M. Medicinal Plants of India, Vol. I. New Delhi: ICMR; 1976.
Lindley J. Flora Medica. New Delhi, India: Ajay Book Service; 1981.
Kurma SR, Mishra SH. Hepatoprotective activity of the whole plant of Fumaria indica. Indian Journalof Pharmaceutical Science. 1997;59:165–170.
Rao KS, Mishra SH. Antihepatotoxic activity of monomethyl fumarate isolated from Fumaria indica. Journal of Ethnopharmacology. 1998;60:207-213.
Rathi A, Srivastava AK, Shirwaikar A, Rawat AKS, Mehrotra S. Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine. 2008;15:470-477.
Pandey VB, Dasguta B, Bhattacharya SK, Lal R, Das PK. Chemistry and pharmacology of the major alkaloid of Fumaria indica. Current Science. 1971; 17:455-457.
Tian Y-H, Kim H-C, Cui1 J-M, Kim Y-C. Hepatoprotective Constituents of Cudrania tricuspidata. Archives of Pharmacal Research. 2005;28:44-48.
Wormser U, Ben Zakine S, Stivelband E, Eisen O, Nyska A. The liver slice system: a rapid in vitro acute toxicity test for primary screening of hepatotoxic agents. Toxicology in Vitro. 1990;4:783–789.
Invittox Protocol No. 42. Liver slice hepatotoxicity screening system. The ERGATT/FRAME Data Bank of in vitro techniques in toxicology. England: INVITTOX; 1992.
Sinha S, Dixit P, Bhargawa S, Devasagayam TPA, Ghaskadbi S. Bark and fruit extracts of Gmelina arborea protect liver cells from oxidative stress. Pharmaceutical Biology. 2006;44:237-243.
Renner K, Amberger A, Konwalinka G, Kofler R, Gnaiger EB. Changes of mitochondrial respiration, mitochondrial content and cell size after induction of apoptosis in leukemia cells. Biochimica et biophysica acta. 2003;1642:115-123.
Wormser U, Ben ZS. The liver slice system-an in vitro acute toxicity test for assessment of hepatotoxins and their antidotes. Toxicology in Vitro. 1990; 4:449–451.
Ohkawa H, Ohoshi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical Biochemistry. 1979; 95:351-358.
Beauchamp C, Fridovich I. Superoxide dismutase: improved assay and an assay applicable to acrylamide gel. Analytical Biochemistry. 1971;44:270-276.
Aebi HE. Catalase. In: Bergmeyer, HU, editors. Methods of Enzymatic Analysis, Vol. 3. Gmbh: Weinhein, Verlagchemie; 1983, p. 277-282.
Goldberg DM. Glutathione reductase. In Bergmeyer HU, editors. Methods of Enzymatic Analysis, Vol 3. Gmbh: Weinhein, Verlagchemie; 1983, p. 277.
Bradford MM. A rapid sensitive method for the quantitation of microgram quantities of protein utilising the principle of dye binding Analytical Biochemistry. 1976;72:248–254.
Frazier JM. In vitro toxicity testing: Application to Safety evaluation. New York: Marcel Dekker, Inc; 1992, p. 45.
Naik RS, Mujumdar AM, Ghaskadbi S. Protection of liver cells from ethanol cytotoxicity by curcumin in liver slice culture in vitro. Journal of Ethnopharmacology. 2004;95:31.