Synthesis and Characterization of Novel Rutin Derivatives with Potential Antioxidant Properties
Keywords:
Malus domestica, Rutin, Reducing power assay - Fe 2 chelating assay - DPPH assay, Ferric thiocyanate assayAbstract
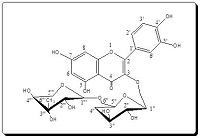
Rutin, a flavonol glycoside isolated from the fruit peels of Malus domestica family Rosaceae, is well known to possess antioxidant activity. This research was conducted in order to synthesize and characterize rutin derivatives and evaluate their antioxidant activities. Four derivatives were synthesized namely, Rutin-oxy-5, 7, 4' acetic acid (2), Rutin -oxy- 5,7, 4' methyl benzoate amide (3), 2'', 2''', 2''''-rutinoxymethyl-3-amino-1 -benzo[5,6-a] pyrimidine-4-one (4) and 5'', 5''', 5''''-tri-p-methoxybenzylideneamino-6-rutinoxy-benzo[5,6-a]-pyrimidin-4(5H)-one (5). Their structures were elucidated using different spectral data (Mass, IR and 1H, 13C NMR). The antioxidant activities of rutin and its derivatives were evaluated by reducing power, Fe+2 chelating, DPPH* and ferric thiocyanate FTC assays. In addition, the results were compared with natural and synthetic antioxidants, such as α- tocopherol, butylated hydroxytoluene (BHT), butylated hydroxyanisole (BHA) and trolox. Rutin and its derivatives were exhibited a strong reducing power, chelating activity on Fe2+ and free radical-scavenging. Antioxidant activity of rutin and its derivatives increased with increased concentrations. Total antioxidant activity of rutin, its derivatives and both standards decreased in the order of rutin > compound (5) > trolox > compound (2) > BHA > compound (4) > compound (3). This study showed that Rutin and its derivatives exhibited antioxidant activity in all tests and could be considered as a source of natural and synthetic antioxidants.
References
Hosseinzadeh H, Nassiri-Asl M. Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest. 2014;37:783-8. 2. Sharma S, Ali A, Ali J, Sahni JK, Baboota S. Rutin: therapeutic potential and recent advances in drug delivery. Expert Opin Investig Drugs. 2013; 22: 1063-79. 3. Choi KS, Kundu JK, Chun KS, Na HK, Surh YJ. Rutin inhibits UVB radiation-induced expression of COX-2 and iNOS in hairless mouse skin: p38 MAP kinase and JNK as potential targets. Arch Biochem Biophys. 2014;559:38-45. 4. Ismail MMF, Ghorab MM, Noaman E, Ammar YA, Heiba HI, Sayed MY. Novel synthesis of pyrrolo[2,3-d]pyrimidines bearing sulfonamide moieties as potential antitumor and radioprotective agents. Arzneimittel-Forschung (Drug Research) 2006; 56(4):301–308. 5. Ghorab MM, Noaman E, Ismail MMF, Heiba HI, Ammar YA, Sayed MY. Novel antitumor and radioprotective sulfonamides containing pyrrolo[2,3-d]pyrimidines. Arzneimittel- Forschung (Drug Research). 2006;56(6):405–413. 6. Ghorab MM, Ragab FA, Noaman E, Heiba HI, Galal M. Synthesis of certain new thieno[2,3-d]pyrimidines as potential antitumor and radioprotective agents. Arzneimittel- Forschung (Drug Research). 2006;56(7):553–560. 7. Heiba HI, Ragab FA, Noaman E, Ghorab MM, Galal M. Synthesis of some novel sulphur containing triazolothienopyrimidines and biscompounds as possible antitumor and radioprotective agents. Arzneimittel-Forschung (Drug Research). 2006;56(8):593–599. 8. Ghorab MM, Osman AN, Noaman E, Heiba HI, Zaher NH. The synthesis of some new sulfur heterocyclic compounds as potential radioprotective and anticancer agents. Phosphorus Sulphur Silicon. 2006;181:1935–1950. 9. Ghorab MM, Osman AN, Noaman E, Heiba HI, Zaher NH. The Utility of isothiocyanato thiophenes in synthesis of thieno[2,3-d]pyrimidine derivatives as possible radioprotective and anticancer agents. Phosphorus Sulphur Silicon. 2006;181:1983–1996. 10. Chauhan PMS, Martins CJA, Horwell DC. Syntheses of novel heterocycles as anticancer agents. Bioorg Med Chem. 2005;13:3513–3518. 11. Cocco MT, Congiu C, Lilliu V, Onnis, V. Synthesis and in vitro antitumoral activity new hydrazinopyrimidine-5-carbonitrile derivatives. Bioorg Med Chem. 2006;14:366–372. 12. Amr AE, Mohamed AM, Mohamed SF, Abdel-Hafez NA, Hammam AG. Anticancer activities of some newly synthesized pyridine, pyrane, and pyrimidine derivatives. Bioorg Med Chem. 2006;14:5481–5488. 13. Abdel-Gawad SM, El-Gaby MSA, Heiba HI, Ali HM, Ghorab MM. Synthesis and radiation stability of some new biologically active hydroquinoline and pyrimido[4,5-b] quinoline derivatives. J Chin Chem Soc. 2005;52:1227–1236. 14. El-Gaby MS, Abdel Gawad SM, Ghorab MM, Heiba HI, Ali HM. Synthesis and biological activity of some novel thieno[2,3-d]quinoline, quinolino[3,2:4,5]thieno[3,2-d]pyrimidine and pyrido[2,3:4,5]thieno[2,3-d]quinoline derivatives. Phosphorus Sulphur Silicon. 2006;181:279–297. 15. Selvi ST, Nadaraj V, Mohan S, Sasi R, Hema M. Solvent free microwave synthesis and evaluation of antimicrobial activity of pyrimido[4,5-b]- and pyrazolo[3,4-b]quinoline. Bioorg Med Chem. 2006;14: 3896–3903. 16. Gawin R, De Clercq E, Naesens L, Stawinska MK. Synthesis and antiviral evaluation of acyclic azanucleosides developed from sulfanilamide as a lead structure. Bioorg Med Chem. 2008;16(18):8379–89. 17. Ezabadi IR, Camoutsis C, Zoumpoulakis P, Geronikaki A, Sokovic M, Glamocilija J, et al. Sulfonamide-1,2,4-triazole derivatives as antifungal and antibacterial agents: Synthesis, biological evaluation, lipophilicity, and conformational studies. Bioorg Med Chem. 2008;16(3):1150–61. 18. Hussein MA. Synthesis of some novel triazoloquinazolines and triazinoquinazolines and their evaluation for anti-inflammatory activity. Medicinal Chemistry Reaserch. DOI 10.1007/s00044-011-9707-0 19. Hussein MA. Synthesis and Biochemical Evaluation of Some Novel Anti-inflammatory Quinazolines. International Journal of Organic and Bioorganic Chemistry. 2011; 1: 12-20. 20. Oyaizu M. Studies on products of browning reaction prepared from glucose amine. Jpn J Nutr 1986; 44:307–314. 21. Decker EA, Welch B. Role of ferritin as a lipid oxidation catalyst in muscle food. J Agr Food Chem 1990; 38:674–683 22. Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. Lebensm Wiss Technol 1995; 28:25–32 23. Takashira M, Ohtake Y. A new antioxidative 1,3-benzodioxole from Melissa officinalis. Planta Med 1998; 64:555–563 24. Osawa T, Namiki N. A novel type of antioxidant isolated from leaf wax of Eucalyptus leaves. Agric Biol Chem 1981; 45:735–742 25. SPSS. (SPSS 15, Inc., Chicago,IL, USA).2012. 26. Yokozawa T, Chen CP, Dong E, Tanaka T, Nonaka GI, Nishioka I. Study on the inhibitory effect of tannins and flavonoids against the 1,1-diphenyl-2-picrylhydrazyl radical. Biochem Pharmacol 1998; 56:213–222. 27. Chung Y, Chen S, Hsu C, Chang C, Chou S. Studies on the antioxidative activity of Graptopetalum paraguayense E. Walther. Food Chem 2005; 91:419–423 28. Baumann J, Wurn G, Bruchlausen FV. Prostaglandin synthase inhibiting O2-radical scavenging properties of some flavonoids and related phenolic compounds. N-Ss Arch Pharmacol 1979; 308:R27–R39. 29. Rice-Evans CA, Miller NJ, Paganga G. Structure antioxidant activity relationship of flavonoids and phenolic acids. Free Radic Biol Med 1996; 20:933–956 30. Cao G, Sofic E, Prior RL. Antioxidant and prooxidant behaviour of flavonoids: structure–activity relationships. Free Rad Biol Med 1997; 22(5):749–760