Immunomodulatory effects of herbal plants plus melatonin on human blood phagocytes
Keywords:
herbal mixture, melatonin, blood phagocyte, phagocytosis, superoxideAbstract
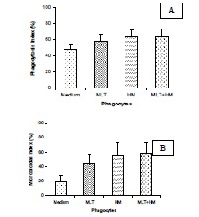
It has been shown a mixture of seven herbal plants was able to trigger cell oxidative mechanism and subsequently inducing cellular activation. Moreover, melatonin hormone has also been shown to perform different actions on the cellular oxidative metabolism. It is possible that the herbal mixture associated with melatonin can activate phagocytes, improving microbicidal activity and further ameliorating resistence to the infections. The aim of this work was to verify in vitro immunomodulatory effects of melatonin and a medicinal plants mixture on blood mononuclear phagocytes (MN). We collected 40 blood samples from normal individuals to obtain the phagocytes. The MN phagocytes were separated by Ficoll-Paque gradient. Preparation of plant extract to obtain the herbal mixture was carried through the process of maceration followed by distillation. Phagocytosis and microbicidal activity of blood phagocytes, treated or not with exogenous superoxide dismutase (SOD), against enterophathogenic Escherichia coli (EPEC) were evaluated by acridine orange method. The herbal mixture and/or melatonin were added to the cell suspensions as immunomodulators. We observed increased phagocytosis and microbicidal activities by blood MN phagocytes in the presence of melatonin or the herbal mixture. The association of both potentiated the functional activity of blood MN phagocytes. Phagocytes previously treated by exogenous SOD had decreased microbicidal activity independently of immunomodulators. These data suggest that the herbal mixture is a potent immunostimulatory agent, and that the interaction between plant and hormones may represent an alternative mechanism of defense against infection, especially in immunosuppressed patients.
References
Asad NR, Assad LMBO, Almeida CEB,
Leitão, A.C. Lethal interaction between
hydrogenperoxide and 0-phenonthroline in
Escherichia coli. Br J Med Biol Res 1994; 27:
-2555.
Honorio-França AC, Carvalho MPSM, Isaac
L, Trabulsi LR, Carneiro-Sampaio MMS.
Colostral mononuclear phagocytes are able to
kill Enteropathogenic Escherichia coli
opsonized with colostral IgA. Scand J
Immunol 1997;46: 59-66.
Honorio-França AC, Launay P, CarneiroSampaio MMS, Monteiro RC. Colostral
neutrophils express IgA Fc receptors (CD89)
lacking y chain association that mediate noninflammatory properties of secretory IgA. J
Leuk Biol 2001; 69: 280-288.
França EL, Pereira Jr A, Oliveira SL,
Honorio-França AC.
Chronoimmunomodulation of melatonin on
bactericidal activity of human blood
phagocytes. Intern J Microbiol 2009; 6:1-15.
Ames BN, Shinnenaga MK, Hagen TM.
Oxidants, antioxidantes, and the degenerative
diseases of agent. Proc Nat Acad Sci 1993;
: 7915-7922.
Tiwari U, Rastogi B, Singh P, Saraf DK, Vyas
SP. Immunomodulatory effects of aqueous
extract of Tridax procumbens in experimental
animals. J Ethnopharmacol 2004; 92: 113-
Atal CK, Sharma ML, Kaul A, Khajuria A.
Immunomodulating agents of plant origin. I:
Preliminary screening. Journal
Ethnopharmacol. 1986; 18:133-134.
Viegas Jr C, Bolzani VS, Barreiro EJ. The
natural products and the modern medicinal
chemistry. Quím Nova 2006; 29:326-337.
Corrêa VSC, Maynié JC, França EL, HonorioFrança AC. Activity of phagocytes in the
Presence of the “Mais Vida” (more life)
Herbal Remedy. Braz J Med Plants 2006; 8:
-32.
França EL, Feliciano ND, Silva KA, Ferrari
CKB, Honorio-França AC. Modulatory Role
of Melatonin on Superoxide Release by
Spleen Macrophages Isolated from AlloxanInduced Diabetic Rats.. Bratisl Med J
;7:163-173.
Dardenne M, Savino W. Control of thymus
physiology by peptidic hormones and
neuropeptides. Immunol Today 1994; 15: 519-
Cassone VM. The pineal gland influences rat
circadian activity rhythms in constant light. J.
Biol. Rhythms 1992; 7: 27-40.
Claustrat B, Brun J, Chazot G. The basic
physiology and pathophysiology of melatonin.
Sleep Med Rev 2005; 9:11–24.
Pandi-Perumal Sr, Trakht I, Srinivasan V,
Spence DW , Maestroni GJM , Zisapel N.
Physiological effects of melatonin: Role of
melatonin receptors and signal transduction
pathways. Prog Neurobiol 2008; 85: 335–353.
Honorio-França AC, Silva KA, Feliciano ND,
Calderon IMP, Rudge MVC, França EL.
Melatonin effects on macrophage in diabetic
rats and the maternal hyperglycemic
implications for newborn rats. Int J Diabet
Metabol 2009; 17:87-92.
Ianãs O, Olinescu R, Bãdescu I. Melatonin
involvement in oxidative processes. Rom J
Endocrinol 1991; 29:147-153.
Finocchiaro LME, Nahmod VE, Launay JM.
Melatonin biosíntesis and metabolism in
peripheral blood mononuclear leucocytes.
Biochem J 1991; 280:727-731.
Harbone, J.B. 1984. Phytochemical Methods.
A guide to Modern Techniques of Plant
Analysis, Second ed. Chapman and Hall,
London, pp. 84-274.
Pawlak J, Singh J, Lea RW, Skwarlo-Sonta K.
Effect of melatonin on phagocytic activity and
intracellular free calcium concentration in
testicular macrophages from normal and
streptozotocin-induced diabetic rats. Mol Cell
Biochem 2005; 275: 207-213.
Bellinati-Pires R, Salgado MM, Hypolito IP,
Grumach AS, Carneiro-Sampaio, MMS.
Aplication of a fluorochrome-lysostaphin
assay to the detection of phagocytic and
bactericidal disturbances in human neutrophils
and monocytes. J Investig Allergol Clin
Immunol. 1995; 5:337-342.
Babu U, Failla ML. Copper status and fuction
of neutrophlis are reversibly depressed in
marginally and severely copper-deficient rats.
J Nutr 1990; 120:1700-1709.
Rêgo, TJA. Fitogeografia das plantas
medicinais no Maranhão. 2nd ed. EDUFMA,
MA, 108-109, 1995.
Calixto, J.B. Efficacy, safety, quality
marketing and regulatory guidelines for herbal
medicines (phytotherapeutic agents). Braz J
Med Biol Res 2000; 32: 179-189.
Hasani-Ranjbar S, Larijani B, Abdollah MA.
Systematic review of Iranian medicinal plants
useful in diabetes mellitus. Arch Med Sci
;.4: 285-292.
Bussmann RW, Meyer AGK, Kuhlman A,
Townesmith A. Herbal mixtures in traditional
medicine in Northern Peru. J Ethnobiol
Ethnomedicine 2010; 6:2-11.
Honorio-França AC; Marins C. Boldrini F,
França EL. Evaluation of hypoglicemic
activity and healing of extract from amongst
bark of "Quina do Cerrado" (Strychnos
pseudoquina ST. HILL). Acta Cir Braz 2008;
: 504-510.
Naser B, Lund B, Henneicke-Von Zepellin
HH, Köhler G, Lehmacher W, Scaglione F. A
randomized, double-blind, placebo-controlled,
clinical dose-response trial of an extract of
Baptisia, Echinacea and Thuja for the
treatment of patients with common cold.
Phytomedicine. 2005;12:715-722.
Besedovsky HO, Del Rey A. Immune-neuroendocrine interactions: Facts and hypotheses.
Endocr Rev 1996; 17: 64–102.
Klepac N, Rudes Z,Klepac R. Effects of
melatonin on plasma oxidative stress in rats
with streptozotocin induced diabetes. Biomed
Pharmacother 2005; 60: 32-35.
Sudnikovich EJ, Maksimchik YZ,
Zabrodskaya SV, Kubyshin VL, Lapshina EA,
Bryszewska M. Melatonin attenuates
metabolic disorders due to streptoxotocininduced diabetes rats. Eur J Pharmacol 2007;
: 180-187.
Manosroi A, Saraphanchotiwitthaya A,
Manosroi J. Immunomodulatory activities of
Clausena excavata Burm. f. wood extracts. J
Ethnopharmacol 2003; 89: 155-160.
Gokhale AB, Damre AS, Saraf MN.
Investigations into the immunomodulatory
activity of Argyreia speciosa. J
Ethnopharmacol 2003; 84: 109-114.
França-Botelho AC, Honorio-França AC,
França EL, Gomes MA, Costa-Cruz JM.
Phagocytosis of Giardia lamblia trophozoites
by human colostral leucocytes. Acta Paediat
; 95: 438-443.
Kang NS, Park SY, Lee SM, Lee BG, Shin
DH, Pyo S. 2002. Modulation of macrophage
function activity by ethanolic extract of larvae
of Holotrichia diomphalia. J Ethnopharmacol
; 79: 89-94.
Traber MG. Cellular and molecular
mechanisms of oxidants and antioxidants. Min
Electrol Metabol 1997; 23: 135-139.
Gort AS, Imlay JA. Balance between
endogenous superoxide stress and antioxidant
defense. J Bacteriol 1998; 180:1402-1410.