The effect of troxerutin on alterations of lipid profile and biochemical enzymes in blood of rats with chronic diabetes
Keywords:
Diabetes, Troxerutin, biochemical enzymes, lipid profile, ratAbstract
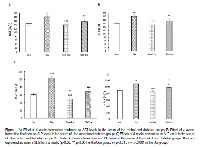
Background & Aims: Diabetes Mellitus (DM) is a progressive disease that leads to complex disorders such as biochemical changes in the blood. The use of medicinal plants are superior to synthetic drugs because of the few side-effects in disease prevention. In this study, we examined the effect of troxerutin on lipid profile and biochemical enzymes in the blood of type 1 diabetic rats. Materials & Methods: 32 male Wistar rats (200-250) were randomly divided into four groups: control, diabetes, control+ troxerutin, diabetes+ troxerutin. Type 1 diabetes was induced by i.p injection of streptozotocin (50 mg/kg) in animals in diabetic groups. Lasted for 4 weeks, oral administration of troxerutin (150 mg/kg) was carried daily for 4 weeks. At the end of study, anesthesia was induced intraperitoneally with sodium pentobarbital (mg / kg 60). Blood samples was collected for measuring lipid profile and biochemical enzymes in blood of rats. Results: Diabete significantly increased LDL, COL, TG and significantly decreased HDL compared to the control group. Treatment diabetic rats with troxerutin for 4 weeks significantly decreased LDL, COL, TG and significantly increased HDL.furthermore, Diabetes significantly increased ALT, AST, LDH, and CPK in blood of rats. Treatment diabetic rats with troxerutin for 4 weeks significantly decreased ALT, AST, LDH, and CPK in blood of rats compared to the control group. Conclusion: Troxerutin improve the lipid profile and reduce biochemical enzymes in blood of diabetic rats.In this way could be useful in reducing the complications of diabetes.
References
Association AD. Diagnosis and classification of diabetes mellitus. Diabetes care. 2004;27:5-10.
Tang WW, Maroo A, Young JB. Ischemic heart disease and congestive heart failure in diabetic patients. Medical Clinics. 2004;88: 1037-61.
Mir M, Arab MR, Shahraki MR, Mashhadi MA, Shahraki Salar M, Sargolzai Aval F. Toxic Effects of Cisplatin on Hepatocytes and Liver Enzymes of Rats. Anatomical Sciences Journal. 2015;12:171-6.
Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889-95.
Celik I, YEĞİNE, ODABAŞOĞLU F. Effect of experimental diabetes mellitus on plasma lactate dehydrogenase and glutamic oxaloacetic transaminase levels in rabbits. Turkish Journal of Biology. 2002;26:151-4.
Suter SL, Nolan JJ, Wallace P, Gumbiner B, Olefsky JM. Metabolic effects of new oral hypoglycemic agent CS-045 in NIDDM subjects. Diabetes care. 1992;15:193-203.
Zhang Z, Wang X, Zheng G, Shan Q, Lu J, Fan S. Troxerutin Attenuates Enhancement of Hepatic Gluconeogenesis by Inhibiting NOD Activation-Mediated Inflammation in High-Fat Diet-Treated Mice. International journal of molecular sciences. 2016;18:31.
Blasig I, Loewe H, Ebert B. Effect of troxerutin and methionine on spin trapping of free oxy-radicals. Biomedica biochimica acta. 1987; 47:S252-5.
Boisseau M, Taccoen A, Garreau C, Vergnes C, Roudaut M, Garreau-Gomez B. Fibrinolysis and hemorheology in chronic venous insufficiency: a double blind study of troxerutin efficiency. The Journal of cardiovascular surgery. 1995;36:369-74.
Kessler M, Ubeaud G, Walter T, Sturm F, Jung L. Free radical scavenging and skin penetration of troxerutin and vitamin derivatives. Journal of dermatological treatment. 2002;13:133-41.
Maurya DK, Salvi VP, Nair CKK. Radioprotection of normal tissues in tumor-bearing mice by troxerutin. Journal of radiation research. 2004;45:221-8.
Tripathi V, Verma J. Different models used to induce diabetes: a comprehensive review. Int J Pharm Sci. 2014;6:29-32.
King AJ. The use of animal models in diabetes research. British journal of pharmacology. 2012; 166:877-94.
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical biochemistry. 1968;25: 192-205.
Erejuwa OO. Management of diabetes mellitus: could simultaneous targeting of hyperglycemia and oxidative stress be a better panacea? International journal of molecular sciences. 2012;13:2965-72.
Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nature clinical practice Endocrinology & metabolism. 2009;5:150-9.
Nesto RW. Beyond Low-Density Lipoprotein. American Journal of Cardiovascular Drugs. 2005;5: 379-87.
Qureshi AA, Sami SA, Khan FA. Effects of stabilized rice bran, its soluble and fiber fractions on blood glucose levels and serum lipid parameters in humans with diabetes mellitus Types I and II. The Journal of nutritional biochemistry. 2002;13:175-87.
Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes. Diabetes care. 2007;30:707-12.
Moayeri H, Oloomi Z. Prevalence of dyslipidemia in children and adolescents with diabetes mellitus type I. Iranian Journal of Pediatrics. 2006;16:171-6.
Pérez A, Wägner AM, Carreras G, Giménez G, Sánchez-Quesada JL, Rigla M. Prevalence and phenotypic distribution of dyslipidemia in type 1 diabetes mellitus: effect of glycemic control. Archives of internal medicine. 2000;160: 2756-62.
Ginsberg HN, Huang LS. The insulin resistance syndrome: impact on lipoprotein metabolism and atherothrombosis. Journal of cardiovascular risk. 2000;7:325-31.
Lewis GF, Steiner G. Acute effects of insulin in the control of VLDL production in humans: implications for the insulin-resistant state. Diabetes care. 1996;19:390-3.
Rader DJ. Effect of insulin resistance, dyslipidemia, and intra-abdominal adiposity on the development of cardiovascular disease and diabetes mellitus. The American journal of medicine. 2007;120:S12-S8.
Sampath S, Karundevi B. Effect of troxerutin on insulin signaling molecules in the gastrocnemius muscle of high fat and sucrose-induced type-2 diabetic adult male rat. Molecular and cellular biochemistry. 2014;395:11.
Lu J, Wu D-m, Zheng Z-h, Zheng Y-l, Hu B, Zhang ZF. Troxerutin protects against high cholesterol-induced cognitive deficits in mice. Brain. 2011;134:783-97.
Chodari L, Mohammadi M, Mohaddes G, Alipour MR, Ghorbanzade V, Dariushnejad H. Testosterone and Voluntary Exercise, Alone or Together Increase Cardiac Activation of AKT and ERK1/2 in Diabetic Rats. Arquivos brasileiros de cardiologia. 2016; 0-.
Pari L, Elangovan P. Troxerutin protects nickel-induced alterations in lipids and plasma lipoproteins in rats. Int. J. Bas. Life Sci. 2013;1: 39-49.
Sadur C, Eckel R. Insulin stimulation of adipose tissue lipoprotein lipase. Use of the euglycemic clamp technique. Journal of Clinical Investigation. 1982;69:1119.
Schmeck-Lindenau H, Naser-Hijazi B, Becker E, Henneicke-von Zepelin H, Schnitker J. Safety aspects of a coumarin-troxerutin combination regarding liver function in a double-blind placebo-controlled study. International journal of clinical pharmacology and therapeutics. 2003;41:193-9.
Arkkila PE, Koskinen PJ, Kantola IM, Rönnemaa T, Seppänen E, Viikari JS. Diabetic complications are associated with liver enzyme activities in people with type 1 diabetes. Diabetes Research and Clinical Practice. 2001;52:113-8.
Adam B, Pentz R, Siegers C, Strubelt O, Tegtmeier M. Troxerutin protects the isolated perfused rat liver from a possible lipid peroxidation by coumarin. Phytomedicine. 2005;12:52-61.
Javadi S, Eftekhari A, Farshid AA. The effects of grape seed oil on histopathological changes of the pancreas, liver and plasma lipids in streptozotocin induced diabetic rats. Urmia medical journal. 2014; 25:605-15.
Baig NA, Herrine SK, Rubin R. Liver disease and diabetes mellitus. Clinics in laboratory medicine. 2001;21:193-207.
Hassanen NH. Protective effect of cinnamon, clove and ginger spices or their essential oils on oxidative stress of streptozotocin-induced diabetic rats. Arab Universities Journal of Agricultural Sciences. 2010;18:137-54.
Celik A, Ersoy OF, Ozkan N, Kayaoglu HA, Ozugurlu F, Cakir EA. Comparison of the effects of troxerutin and heparinoid on flap necrosis. Journal of Plastic, Reconstructive & Aesthetic Surgery. 2010;63:875-83.
Davison GW, George L, Jackson SK, Young IS, Davies B, Bailey DM. Exercise, free radicals, and lipid peroxidation in type 1 diabetes mellitus. Free Radical Biology and Medicine. 2002;33:1543-51.
Masjedi F, Gol A, Dabiri S, Javadi A. Preventive effect of garlic on histopathology of liver and markers of hepatic injury in streptozotocin-induced diabetic Rats. Iranian Journal of Endocrinology and Metabolism. 2009;11:433-41.
Liu CM, Ma JQ, Lou Y. Chronic administration of troxerutin protects mouse kidney against D-galactose-induced oxidative DNA damage. Food and Chemical Toxicology. 2010;48:2809-17.
Lu J, Wu Dm, Hu B, Cheng W, Zheng Yl, Zhang Zf. Chronic administration of troxerutin protects mouse brain against D-galactose-induced impairment of cholinergic system. Neurobiology of learning and memory. 2010;93: 157-64.