Screening of some plant materials used in South-West Algerian traditional medicine for their antibacterial activity
Keywords:
Medicinal Plants, antibacterial activity, antibiotics susceptibilityAbstract
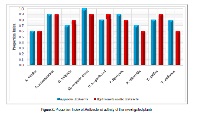
The initial introduction of new medicinal agents into the health care system sometimes, requires information beyond that is recorded in libraries relying instead, on reports available through traditions and healers within a society. This paper explored the antibacterial activity of aqueous and hydromethanolic extracts of nine folkloric medicinal plant from Bechar region (southwest Algeria) namely: A. nardus, A. schoenanthus, G. vulgaris, two species of H. scoparia green & red, P. laevigata, R. tripartita, T. gallica and T. nudatum, frequently used in the local traditional medicine. The antibacterial activity of different extracts were evaluated by using disc diffusion method agar and antibiotics susceptibility of ten selected microorganisms: seven reference strains, Bacillus cereus, Enterococcus faecalis, Staphylococcus aureus, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Salmonella typhi, and three clinically isolated strains, Escherichia coli (Urinary Tract Infection), Escherichia coli (Vaginal Infection) and Staphylococcus aureus (Skin Infection). The maximum antibacterial activity was recorded against the gram negative reference strains Pseudomonas aeruginosaand Escherichia coli with a maximum inhibition diameter of 15.6 ± 0.5 and 15.0 ± 1.4 mm respectively displayed by the aqueous extract of T. gallica, followed by the activity detected by the hydromethanolic extract of R. tripartita against the gram negative reference strain Pseudomonas aeruginosa (14.6±1.2 mm) and the aqueous and hydromethanolic extracts of R. tripartita against the gram negative reference strains Pseudomonas aeruginosa and Escherichia coli with a maximum inhibition diameter of 14.3 ± 2.0 and 14.3±0.5 mm, respectively. According to the present study, H. scoparia red, P. laevigata, R. tripartita, and T. gallicacan be served as broad spectrum antibiotic and used as a potent source of natural antibacterial agents by replacing commercially available synthetic drug that may have a large number of side effects.
References
El-Bashiti TA, Abou Elkhair E and Abu Draz WS. The antibacterial and synergistic potential of some Palestinian plant extracts against multidrug resistant Staphylococcus aureus. J. Med. Plants Stud. 2017; 5(2):54-65.
Kumar A, Dudeja S, Chauhan R, Sunena H, Beniwal V, Chhokar V and Kumar A. Antimicrobial activity of ethno-medicinal plants against cariogenic pathogens. J. Med. Plants Stud. 2016;4(3):283-290.
Manaf SR and Daud HM. Screening of phytochemical properties and antimicrobial activity of Malaysian medicinal plants against aquatic bacteria. Malaysian. J. Microbiol. 2016;12(3):284-290.
Dathar V and Afrojahan A. Effect of Coleus Amboinicus Leaf Extract and Oil on Clinical Isolates of Pseudomonas and Proteus. Int. j. Appl. Pharm. Biol. Res. 2017;2(2): 3939-4747.
Kage DN, Seetharam YN and Malashetty VB. In Vitro Antibacterial Property and Phytochemical Profile of Trichosanthes cucumerina L. Var. cucumerina. Adv. Nat. Appl. Sci. 2009;3(3):438-441.
Mahon C and Manuselis G. Textbook of Diagnostic Microbiology, 3rd ed. Philadelphia: W.B. Saunders. 2006.
Frerichs GN and Millar SD. Manual for the isolation and identification of fish bacterial pathogens. 1st ed. Stirling, Scotland: Pisces Press. 1993.
Joseph Mc Farland MD. The nephelometer : An instrument for estimating the number of bacteria in suspensions used for calculating the opsonic index and for vaccines. J Am. Med. Assoc. 1907; XLIX (14):1176-1178.
Hindler JA and Jorgensen JH. Procedures in antimicrobial testing. In: Mahon, C.R. and Manuselis, G., editors. Textbook of Diagnostic Microbiology. 2nd ed. Philadelphia: W.B. Saunders. 2000.
Gupta N, Mittal M, Parashar P, Mehra V and Khatri M. Antibacterial Potential of Elletariacardamomum, Syzygium aromaticum and Piper nigrum, their synergistic effects and phytochemical determination. J. Pharm. Res. 2014;8(8):1-7.
Casella S, Leonardi M, Melai B, Fratini F and Pistelli L. The role of diallyl sulfides and dipropyl sulfides in the in vitro antimicrobial activity of the essential oil of garlic, Allium sativum L., and leek, Allium porrum L. Phytother. Res. 2013; 27(3):380-383.
Carson CF and Riley TV. Antimicrobial activity of the major components of the essential oil of Melaleuca alternifolia. J. Appl. Bacteriol. 1995;78(1):264-269.
Bauer AW, Kirby WM, Sherris JC. And Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 1966;45(1):493-496.
Borgio JF, Thorat PK and Lonkar AD. Antimycotic and antibacterial activities of Gynandropsis pentaphylla DC. Extracts and its phytochemical studies. Int. J. Microbiol. 2008;5(1):1-6.
Singh B, Sahu PM and Sharma MK. Anti- inflammatory and antimicrobial activities of triterpenoids from Strobilanthes callosus Necs. Phytomedicine 2002;9(1):355-359.
Forbes BA, Sahm DF and Weissfeld AS. Bailey & Scott's Diagnostic Microbiology, 12th ed. Missouri: Mosby Elsevier. 2007.
Monica C. Medical Laboratory manual for Tropical countries 1991;11:60-63.
Gupta D, Dubey J and Kumar M. Phytochemical analysis and antimicrobial activity of some medicinal plants against selected common human pathogenic microorganisms. Asian Pac. J. Tropical Disease. 2016;6(1):15-20.
Srinivasahan V and Durairaj B. Antioxidant and free radical scavenging effect of Morinda citrifolia fruit extract. Int. J Pharma. Pharmaceut. Sci. 2014; 6(4):55-59.
Singh D, Sati SC and Sati MD. In vitro antimicrobial activity of Himalayan medicinal plant Pholidota articulate. Int. J. Herb. Med. 2016;4(6):01-03.
Rubalakshmi G, Nirubama K and Prabhakaran S. Structural Delving and Insilico Analysis of Proteins of Rhinacanthus Nasutus: An Indigenous Medicinal Plant. J. Adv. Appl. Sci. Res. 2016;(6):1-16.
Ram J, Moteriya P and Chanda S. Phytochemical screening and reported biological activities of some medicinal plants of Gujarat region. J. Pharma. Phytochem. 2015;4(2):192-198.
Padalia H and Chanda S. Comparative phytochemical analysis of aerial parts of A. procumbeans, F. dichotoma, S. sponteneum, S. nigra and T. angustifolia. J. Phcog. Phytochem. (In press) 2015;4(2).
Moteriya P, Satasiya R and Chanda S. Screening of phytochemical constituents in some ornamental flowers of Saurashtra region. J. Phcog. Phytochem. 2015;3(5):112-120.
Abeysinghe PD, Pathirana RN and Wanigatunge RP. Evaluation of antibacterial activity of different mangrove plant extracts. Ruhuna J. Sci. 2012;1(1):345-349.
Karmegam N, Karuppusamy S, Prakash M, Jayakumar M and Rajasekar K. Antibacterial potency and synergistic effect of certain plant extracts against food-borne diarrheagenic bacteria. Int. J. Biomed. Pharm. Sci. 2008;2(2):88-93.
Edayadulla N and Ramesh P. Antibacterial activity of various stem extracts of Dalbergia coromandeliana. Asian Pac. J. Trop. Biomed. 2012;2(l3):1388-1391.
Al-Daihan S, Al-Faham M, Al-shawi N, Almayman R, Brnawi A, Zargar S. Antibacterial activity and phytochemical screening of some medicinal plants commonly used in Saudi Arabia against selected pathogenic microorganisms. J. King Saud. Univ. Sci. 2013;25(2):115- 20.
Darout IA, Christy AA, Skaug N and Egeberg PK. Identification and quantification of some potentially antimicrobial anionic components in Miswak extract. Indian J. Pharmacol. 2000;32(1):1-4.
Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12(4):564-82.
Singh N, Gupta S and Rathore V. Comparative Antimicrobial Study of Ethanolic Extract of Leaf and Rhizome of Curcuma longa Linn. Pharm. J. 2017;9(2):208-212.