Effect of phenolic extracts of Algerian medicinal plants on the bacterial growth and adherence of Staphylococcus aureus and Bacillus cereus pathogens responsible of food poisoning.
Keywords:
food poisoning, S. aueus; B. cereus, A. herba alba, T. capitatus, AntioxydantAbstract
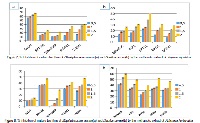
Artemisia, one of the larger genera in the family Asteraceae, comprises from 200 to more than 500 taxa at the specific or subspecific level. Artemisia herba-alba Asso (Shih) is grown in North Africa and certain parts of Asia and Middle East. It is one of the most widely used plants in the Algerian folk medicine. The antioxidant and free radical scavenging activities of the methanolic extracted materials were tested together with their antibacterial effects against isolated bacterial strains causing food poisoning. In summary, A. herba-alba Asso represent a good phenolic and flavonoid content (161, 64 mg/g and 16,83mg/g, respectively) in comparison with T. capitatus L which represent a phenolic content of 131, 48 mg/g and 14, 96 of flavonoid content. The results shows also that the methanolic extracts of the two plants possess a strong antioxidant (DPPH, FRAP assays) and antibacterial activities, which supports their ethnopharmacological use and A. herba alba represent the highest values (IC 50: 2, 35 mg/ml and OD 1, 13 at 700nm). Our results show the interest of A. herba-alba Asso and T. capitatus L, among other medicinal plants, in search of new chemo-preventive agents against biofilm and planktonic growth of food spoilage pathogens. Further studies are envisaged to target the most interesting molecules responsible for these activities. It is concluded that organic extracts from Artemisia herba alba Asso, exert strong antioxidant activities which are related to their polyphenol contents.
References
. Seo KS, Bohach GA. Staphylococcus aureus. InFood Microbiology: Fundamentals and Frontiers, Third Edition American Society of Microbiology.2007;493-518. [2]. Griffiths MW, Schraft H. Bacillus cereus food poisoning. In D. O. Cliver, & H. P. Riemann (Eds.), Foodborne diseases (2nd Ed.). (261e270) London: Academic Press. 2002. [3]. Feuerstein I, Muller D, Hobert K, Danin A, Segal R. The constituents of essential oilsfrom Artemisia herba alba population of Israel and Sinai. Phytochemistry 1986; 25:2343-2347. [4]. Akrout A, El Jani H, Amouri S, Neffati M. Screening of antiradical and antibacterial activities of essential oils of Artemisia campestris L., Artemisia herba alba Asso and Thymus capitatus Hoff. Et Link. Recent Research in Science and Technology. 2010; 2(1): 29-39. [5]. Rice-Evans CA, Miller NJ, Paganga G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radical Biology and Medicine, 1996; 20, 933-956. [6]. Van der Veen S, Abee T. Mixed species biofilms of Listeria monocytogenes and Lactobacillus plantarum show enhanced resistance to benzalkonium chloride and peracetic acid. Int. J. Food Microbiol. 2011; 144:421–431. [7]. FDA Bacteriological Analytical Manual: Staphylococcus aureus, In: AOAC International, 8th rev. ed., Gaithersburg, MD, 1998; 12.01-12.05 [8]. Yousef AE, Carlstrom C. Staphylococcus aureus. In: Food Microbiology: A Laboratory Manual, A Wiley- Interscience publication. 2003. [9]. Cappucino JG, Sherman N. Microbiology A Laboratory Manuel Pearson Education (Singapore) Indian Branch. New Delhi. 2004. [10]. Collins CH, Lyne PM, Grange JM. Collins and Lyne’s microbiological methods. 7thed. London: Arnold. 2001. [11]. Guttman DM, Ellar DJ. Phenotypic and genotypic comparisons of 23 strains from the Bacillus cereus complex for a selection of khown and putative B. thuringesis virulence factors. FFMS Microbial LeH. 2000; 118(1) 7-13. [12]. Gudmundsdo BK. Comparison of extracellular proteases produced by Aeromonas salmonicida strains isolated from various fish species. Applied Bacterioly. 1996; 80, 105–113. [13]. Monica C. Medical Laboratory manual for Tropical countries. ELBS, 1991; 60–63. [14]. Cotter JJ, gara JP, Mack D, Casey E. Oxygen-mediated regulation of biofilm development is controlled by the alternative sigma factor σβ in Staphylococcus epidermidis. Appl Environ Microbiol 2009; 75(1):261–4. [15]. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. American Journal of Enology and Viticulture. 1965; 16, 144–158. [16]. Liu X, Zhao M, Wang J, Yang B Jiang Y. Antioxidant activity of methanolic extract of emblica fruit (Phyllanthus emblica L.) from six regions in China. Journal of Food Composition and Analysis 2008; 21, 219–228. [17]. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autoxidation of soybean oil in cyclodextrin emulsion. Journal of Agricultural and Food Chemistry. 1992; 40, 945-948. [18]. Oyaizu M. Studies on products of browning reaction. The Japanese Journal of Nutrition and Dietetics. 1986; 44 (6): 307-15. [19]. Valgas C, Souza SMD. E.F.A. Smânia and A. Smânia Jr: Screening methods to determine antibacterial activity of natural products. Brazilian Journal of Microbiology. 2007; 38:369–380. [20]. Christensen GD, Purisi JT, Bisno AL, Sineson WA and Beachey EH. Characterization of clinically significant strains of coagulase-negative Staphylococci. J. Clin. Microbiol. 1983; 18: 258-264. [21]. Powers EM, Lawyer R, Masuoka Y. Microbiology of processed spices. J. Milk Food Technol. 1975; 38, 683–687. [22]. Banerjee M, Sarkar PK. Growth and enterotoxin production by sporeforming bacterial pathogens from spices. Food Control. 2003; 15(6), 491-496. [23]. Le Loir Y, Baron F, Gautier M. Staphylococcus aureus and food poisoning. Genet Mol Res. 2003; 2 (1), 63-76 [24]. Zschöck M, Botzelr D, Blöcher S, Sommerhäuser J, Hamann HP. Detection of genes for enterotoxins (ent) and toxic shock syndrome toxin-1 (tst) in mammary isolates of Staphylococcus aureus by polymerase chain reaction. Int Dairy J. 2000; 10, 569-574. [25]. Tamarapu, S, McKillip JL, Drake M. Development of a multiplex polymerase chain reaction assay for detection and differentiation of Staphylococcus aureus in dairy products. J. Food Prot. 2001; 64, 664–668. [26]. Wieneke AA, Roberts D, Gilbert RJ. Staphylococcal food poisoning in the United Kingdom, 1969–1990 Epidemiol. Infect. 1993; 110, 519–531. [27]. Ceuppens S, Boon N, Uyttendaele M. Diversity of Bacillus cereus group strains is reflected in their broad range of pathogenicity and diverse ecological lifestyles. FEMS Microbiol Ecol. 2013; 84: 433-50. [28]. Yobouet BA, Kouame-Sina SM, Dadi ´ e A, Makita K, Grace D, ´ Dje KM, et al. Contamination of raw milk with ` Bacillus cereus from farm to retail in Abidjan, Coted ’Ivoire and possible health implications. Dairy Sci Technol. 2014; 94: 51-60. [29]. Lee JH. Methicillin (oxacillin)-resistant Staphylococcus aureus strains isolated from major food animals and their potential transmission to humans. Appl. Environ. Microbiol. 2003; 69:6489 –6494. [30]. Cosentino S, Mulargia AF, Pisano B, Tuveri P, Palmas F. Incidence and biochemical characteristics of Bacillus flora in Sardinian dairy products. Int J Food Microbiol. 1997; 38:235e8. [31]. Molva C, Sudagidan M, Okuklu B. Extracellular enzyme production and enterotoxigenic gene profiles of Bacillus cereus and Bacillus thuringiensis strains isolated from cheese in Turkey. Food Control. 2009; 20:829-34. [32]. De Jonghe V, Coorevits A, De Block J, Coillie EV, Grijspeerdt K, Herman L, et al. Toxinogenic and spoilage potential of aerobic spore-formers isolated from raw milk. Int J Food Microbiol. 2010; 136:318-25. [33]. Kloos WE, Musselwhite MS. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl. Microbiol. 1975; 30:381–385. [34]. Kaur C, Kapoor HC. Antioxidant activity and total phenolic content of some Asian vegetables. Int. J. Food. Sci. Technol. 2002; 37, 53–161. [35]. Ross KA, Beta T, Arntfield SD. A comparative study on the phenolic acid identified and quantified in dry beans using HPLC as affected by different extraction and hydrolysis methods. Food Chemistry. 2009; 113, 336–344. [36]. Rødtjer A, Skibsted LH, Andersen ML. Antioxidative and prooxidative effect of extracts made from cherry liqueur pomace. Food Chemistry. 2006; 99, 6-14. [37]. Bourgou S, Ksouri R, Bellila A, Skandrani I, Falleh H, Marzouk B. Phenolic composition and biological activities of Tunisian Nigella sativa L. shoots and roots. Comptes Rendus Biologie. 2008; 331, 48–55. [38]. Bounatirou S, Smiti S, Miguel MG, Faleiro L, Rejeb MN, Neffati M, Costa MM, Figueiredo A, Barroso JG, Pedro LG. Chemical composition antioxidant and antibacterial activities of the essential oils isolated from Tunisian Thymus capitatus Hoff et Link. Food Chemistry. 2007; 105, 146–155. [39]. Miguel G, Simoes M, Figueiredo AC, Barroso JG, Pedro LG, Carvalho L. Composition and antioxidant activities of the essential oils of Thymus caespititius, Thymus camphoratus and Thymus mastichina. Food Chemistry. 2004; 86, 183–188. [40]. Espin JC, Garcia-Conesa, MT, Tomas-Barberan FA. Nutraceuricals: Facts and fiction. Phytochemistry. 2007; 68, 2986–3008. [41]. Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. Food Science and Technology. 2007; 40, 344–352. [42]. Das NP, Pereira TA. Effects of flavonoids on thermal autooxidation of Palm oil: structure- activity relationship. J. Am. Oil Chem. Soc.1990; 67: 255-258. [43]. Matkowski A. Plant in vitro culture for the production of antioxidants - A review. Biotechnol. Adv. 2008; 26: 548-560. [44]. Shimoi K, Masuda S, Shen B, Furugori B, Kinae N. Radioprotective effect of antioxidant plant flavonoids in mice. Mutat. Res, 1996; 350: 153-161. [45]. Braga PC, Sasso MD, Culici M, Galastri L, Marceca MT, Guffanti EE. Antioxidant potential of thymol determined by chemiluminescence inhibition in human neutrophilis and cell free systems. Pharmacology. 2006; 76, 61–68. [46]. Braga PC, Dal Sasso M, Culici M, Bianchi T, Bordoni L, Marabini L. Anti- inflammatory activity of thymol: inhibitory effect on the release of human neutrophil elastase. Pharmacology. 2006; 77, 130–136. [47]. Khanuja SPS, Srivastava S, Shasney AK, Darokar MP, Kumar TRS, Agrawal KK, Ahmed A, Patra,NK, Sinha P, Dhawan S, Saikia D, Kumar S. Formulation comprising thymol useful in the treatment of drug resistant bacterial infections. US Patent. 2004; 6, 795–824. [48]. Pietta PG. Flavonoids as antioxidants. J. Nat. Products. 2000; 63: 1035-1042. [49]. Kiselova Y, Ivanova D, Chervenkov T, Gerova D, Galunska B, Yankova T. Correlation between the in vitro antioxidant activity and polyphenol content of aqueous extracts from bulgarian herbs. Phytotherapy Research. 2006; 20(11), 961- 965. [50]. Djeridane A, Yousfi M, Nadjemi B, Boutassouna D, Stocker P, Vidal N. Antioxidant activity of some Algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006; 97, 654–660. [51]. Al Mustafa AH, Al Thunibat OY. Antioxidant activity of some Jordanianplants used tradionally for treatment of diabetes. Pak. J. Biol. Sci. 2008; 11, 351–358. [52]. Yashphe J, Segal R, Breuer A, Erdreich-Naftali G. Antibacterial activity of Artemisia herba alba. J. Pharm. Sci. 1979; 68(7): 924-925. [53]. Kaur S, Shinna GK. Antibacterial activity of volatile oils and their important constitutents from some indigenous plants. Indian J. Phys. Nat. Sci. 1982; 15: 43-47. [54]. Middleton E Jr, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol. Rev. 2000; 52: 673-751. [55]. George FOA, Ephraim RN, Obasa SO, Blankole MO. Antimicrobial properties of some plant extracts on organisms associated with fish spoilage. AJMR. 2009; 6 (2),12-17. [56]. Gur S, Balik DT and Gur N. Antimicrobial activities and some fatty acids of Turmeric, ginger root and Linseed used in the treatment of infectious diseases. World Journal of Agricultural Sciences. 2006; 2(4): 439-442. [57]. Teh KH, Flint S, Palmer J, Andrewes P, Bremer P, Lindsay D. . Biofilm an unrecognised source of spoilage enzymes in dairy products? International Dairy Journal. 2014; 34, 32–40. [58]. Mizan MFR, Jahid IK, Ha SD. Microbial biofilms in seafood: a food-hygiene challenge. Food Microbiology. 2015; 49, 41–55. [59]. Bai AJ, Vittal RR. Quorum sensing inhibitory and anti-biofilm activity of essential oils and their in vivo efficacy in food systems. Food Biotechnology. 2014; 28, 269–292. [60]. Brooks JD, Flint SH. Biofilms in the food industry: problems and potential solutions. Int. J. Food Sci. Technol. 2008; 43:2163–2176. [61]. Rode TM, Langsrud S, Holck A, Møretrø T. Different patterns of biofilm formation in Staphylococcus aureus under food-related stress conditions. Int. J. Food Microbiol. 2007; 116:372–383.