Bone regenerative effect of aqueous Cynanchum wilfordii extract in receptor activator of nuclear factor-κB ligand-induced osteoclast differentiation and estrogen deficiency-induced osteoporosis
Keywords:
Bone mineral density, Cynanchum wilfordii aqueous extract, estrogen deficiency-induced osteoporosis, osteoclast differentiationAbstract
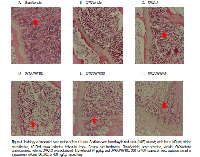
Osteoporosis increases with age, most frequently in postmenopausal women because of reduced ovarian hormone levels. Furthermore, estrogen deficiency impairs trabecular metaphyseal bone. Although efficacious, long-term hormone replacement therapy (HRT) has estrogen-like side effects including breast and endometrial cancers, and non-hormonal or herbal therapies may be safer alternatives. Therefore, the aim of this study was to investigate the effects of aqueous extracts of Cynanchum wilfordii (CWW) on receptor activator of nuclear factor-κ B (NF-κ B) ligand (RANKL)-induced osteoclast differentiation in vitro and ovariectomy-mediated osteoporosis in vivo. CWW inhibited RANKL-induced osteoclast formation and tartrate-resistant acid phosphatase (TRAP) activity in primary mouse bone marrow-derived cells. We investigated the osteoprotective effect of CWW in an ovariectomized (OVX) Sprague-Dawley rat model treated with vehicle (OVX/vehicle), 17β-estradiol (OVX/E2), or three CWW doses (100, 200, and 400 mg/kg). After a 24-week treatment, the body and uterus weights were not affected except in the OVX/E2 group. Additionally, bone mineral density (BMD) and histological analyses showed that the BMD of the femurs of CWW400-treated rats was significantly higher than that of the OVX/vehicle rats, and comparable to that of the OVX/E2 group rats. Serum levels of bone turnover markers alkaline phosphatase (ALP), osteocalcin, collagen type I C-telopeptide, and TRAP significantly decreased in the CWW400 group. Our results show that compared to the vehicle, CWW had a significant anti-osteoporotic effect in the OVX model. Taken together, CWW exhibited inhibitory effects on osteoclastogenesis in vitro, and we confirmed its in vivo efficacy in the prevention of osteoporosis.
References
. Raisz L. Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest, 2005; 115: 3318-3325.
. Papachroni K, Karatzas D, Papavassiliou K, Basdra E, Papavassiliou A. Mechanotransduction in osteoblast regulation and bone disease. trends Mol Med, 2009; 15: 208-216.
. Asagiri M, Takayanagi H. The molecular understanding of osteoclast differentiation. Bone, 2007; 40: 250-64.
. Arai, Miyamoto T, Ohneda O, Inada T, Sudo T, Brasel K, Miyata T, Anderson DM, Suda T. Commitment and differentiation of osteoclast precursor cells by the sequential expression of c-fms and receptor activator of nuclear factor kappa B (RANK) receptors. J Exp Med, 1999; 19D : 1741-1754.
. Kirstein B, Chambers TJ, Fuller K. Secretion of tartrate resistant acid phosphatase by osteoclasts with resorptive behavior. J cell biochem, 2006; 98: 1085-1094.
. Hadjidakis D, Androulakis I. Bone remodeling. Ann N Y Acad Sci, 2006; 1092: 385-396.
. Jung K, Lein M. Bone turnover markers in serum and urine as diagnostic, prognastic and monitoring biomarkers of bone metastasis. Biochim Biophys Acta, 2014; 1846: 425-438.
. Ferreira A, Alho I, Casimiro S, Costa L. Bone remodeling markers and bone metastases: From cancer research to clinical implications. BoneKEy Reports, 2015; 4: 1-9.
. Syed F, Khosla S. Mechanisms of sex steroid effects on bone. Biochem Biophys Res Commum, 2005; 328: 688-696.
. Turner R, Riggs B, Spelsberg T. Skeletal effects of estrogen. Endocr Rev , 1994; 15: 275-300.
. Hukkanen M, Platts LA, Lawes T, Girgis SI, Konttinen YT, Goodship AE, Maclntyre I, Polak JM. Effect of nitric oxide donor nitroglycerin on bone mineral density in a rat model of estrogen deficiency-induced osteopenia. Bone, 2003; 32: 142-149.
. Shin C, Wu Y, Lin W. Ameliorative effects of Anoectochilus formosanus extract on osteopenia in ovariectomized rats. J Ethnopharmacol, 2001; 77: 233-238.
. Ahldorg H, Johnell O, Turner CH, Rannevik G, Karlsson M. Bone loss and bone size after menopause. N Engl J Med, 2006; 349: 327-334.
. Stevenson J. Justification for the use of HRT in the long-term prevention of osteoporosis. Maturitas, 2005; 51: 113-126.
. prelevic G, Kocjan T, Markou A. Hormone replacement therapy in postmeno-pausal women. Minerva Endocrinol, 2005; 30: 27-36.
. Banu J, Varela E, Fernandes G. Alternative therapies for the prevention and treatment of osteoporosis. Nutrition, 2012; 70: 22-40.
. Yang SB, Lee SM, Park J, Lee TH, Baek N, Park H, Lee H, Kim J. Cynandione A from Cynanchum wilfordii Attenuates the Production of Inflammatory Mediators in LPS-Induced BV-2 Microglial Cells via NF-κB Inactivation. Bio Pharm Bull, 2014; 37: 1390-1396.
. Chang A, Kwak B, Yi K, Kim J. The Effects of Herbal Extract (EstroG-100) on pre-, peri-and post-Menopausal Women: A Randomized Double-blind, Placebo-controlled study. Phytother Res, 2012; 26: 510-516.
. Takahashi N, Yamada H, Yosiki S, Roodman G, Mundy G, Jones S, Boyde A, Suda T. Osteoclast-like cell formation and its regulation by osteotropic hormones in mouse bone marrow cultures. Endocrinology, 1998; 122: 1373-1382.
. Jarvinen T, Sievanen H, Kannus P, Jarvinen M. Dual-energy X-ray absorptiometry in predicting mechanical characteristics of rat femur. Bone, 1998; 22: 551-558.
. Kastl S, Sommer T, Klein P, Hohenberger W, Engelke K. Accuracy and precision of bone mineral density and bone mineral content in excised rat humeri using fan beam dual-energy X-ray absorptiometry. Bone, 2002; 30: 243-246.
. Hidaka S, Okamoto Y, Yamada Y, Kon Y, Kimura T. A Japanese herbal medicine, Chujoto, has a beneficial effect on osteoporosis in rats. Phytother. Res, 1999; 13: 14-19.
. Ross F, Teitelbaum S. Alphavbeta3 and macrophage colony-stimulating factor: partnersin osteoclast biology. Immunol Rev, 2005; 208: 88-105.
. Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology, 2001; 142: 5050-5055.
. Levine J. Effective strategies to identify postmenopausal women at risk for osteoporosis. Geriatrics, 2007; 62: 22–30.
. Hoegh-Andersen P, Tanko L, Andersen T, Lundberg C, Mo J, Heegaard A, Delaisse J, Christgau S. Ovariectomized rats as a model of postmenopausal osteoarthritis: Validation and application. Arthritis REs Ther, 2004; 6: R169-R180.
. Jee W, Yao W. overview: animal models of osteopenia and osteoporosis. J Musculoskel Neuron Interact, 2001; 1: 193-207.
. Lelovas P, Xanthos T, Thoma S, Lyritis G, Dontas I. The laboratory rat as an animal model for osteoporosis research. Comp Med. 2008; 58: 424-430.
. Dang Z, van Bezooijen R, Karperien M, Papapoulos S, Lowik C. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J Bone Miner Res, 2002; 17: 394-405.
. Devareddy L, Khalil D, Smith B, Lucas E, Soung D, Marlow D, Arjmandi B. Soy moderately improves microstructural properties without affecting bone mass in an ovariectomized rat model of osteoporosis. Bone, 2006; 38: 686-693.
. Hewitt S, Korach K. Oestrogen receptor knockout mice: Roles for oestrogen receptors alpha and beta in reproductive tissues. Reproduction, 2003; 125: 143-149.
. Kim J, Cho H, Kim Y. The role of estrogen in adipose tissue metabolism: insights into glucose homeostasis regulation. Endocr J. 2014; 61: 1055-1067.
. Han S, Lee T, Jang J, Song H, Hong S, Kim Y, Han B. Mixture of extracts of Cynanchum wilfordii and phlomis umbrosa Turcz. does not have an estrogenic effect in ovariectomized rats. Korean J food SCi technol, 2015; 47: 667-675.
. Park J, Ha S, Kang T, Oh M, Cho M, Lee S, Park J, Kim S. Protective effect of apigenin on ovariectomy-induced bone loss in rats. Life Sci, 2008; 82: 1217-1223.
. Cummings S, Bates D, Black D. Clinical use of bone densitometry: scientific review. JAMA, 2002; 288: 1889-1897.
. Bahlous A, Kalai E, Hadj Salah M, Bouzid K, Zerelli L. Biochemical markers of bone remodeling: recent data of their applications in managing postmenopausal osteoporosis. Tunis Med. 2006; 84: 751–757.
. McComb R, Posen S. Alkaline phosphatase. New York: Plenum Press.1979
. Hlaing T, Compston J. Biochemical markers of bone turnover-uses and limitations. Ann Clin Biochem, 2014; 51: 189-202.