Effects of Opuntia ficus-indica flower extract WS 1261 on mental and psychic function in subsyndromal fatigue – A pilot study
Keywords:
Fatigue, Flavonoids, Mental and psychic function parameters, Opuntia ficus-indica, Proof-of-Principle study, SokatinAbstract
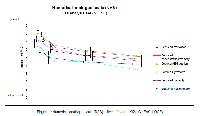
Background Traditional use, chemical constituents, and results of prior animal studies indicate alleviating effects of Opuntia ficus-indica flowers extract on subsyndromal fatigue symptoms in humans Methods 50 healthy subjects of either gender aged 30 to 60 years with subsyndromal fatigue were enrolled in this open-label monocentric single arm proof-of-principle study. 500mg of a hydro-alcoholic extract from Opuntia ficus-indica flowers were administered daily over 8 weeks. Mental and psychic function parameters were assessed by numerical analogue scale of Subjective Fatigue Symptoms, Multidimensional Fatigue Inventory 20, Sheehan Disability Scale, Short Form Health Survey 36, Fatigue Impact Scale, and Global Self-Assessment. Also, health-related incidents, laboratory parameters and vital signs were measured during the intervention period. Results Improvement of fatigue symptoms could be shown in most measurements after week 1 and continued further throughout the trial. 70.0% of subjects reported global improvement of their complaints at end of study. Conclusions A consistent and positive effect of Opuntia ficus-indica flowers extract on symptoms of fatigue could be demonstrated. Improvement in all mental and psychic functions could be measured, most of them statistically significant.
References
Benayad Z, Martinez-Villaluenga C, Frias J, Gomez-Cordovesa C, NourEddine ES. Phenolic composition, antioxidant and anti-inflammatory activitiesof extracts from Moroccan Opuntia ficus-indica flowers obtained by different extraction methods. Ind Crop Prod 2014; 62:412–420 2. Feugang JM, Konarski P, Zou D, Stintzing FC, Zou C. Nutritional and medicinal use of Cactus pear (Opuntia spp.) cladodes and fruits. Front. Biosci 2006; 11:2574–2589 3. Ghazi Z, Ramdani M, Tahri M, Rmili R, Elmsellem H, El Mahi B, Fauconnier ML. Chemical Composition and Antioxidant Activity of seeds oils and fruit juice of Opuntia Ficus Indica and Opuntia Dillenii from Morocco J. Mater. Environ. Sci 2015; 6 (8):2338-2345 4. Stintzing FC, Herbach KM, Mosshammer MR, Carle R, Yi W, Sellappan S, Akoh CC, Bunch R, Felker P: Color, betalain pattern, and antioxidant properties of cactus pear (Opuntia spp.) clones. J Agric Food Chem 2005; 53(2):442-51 5. Galati E, Mondello M, Giuffrida D, Dugo G, Miceli N, Pergolizzi S. Chemical characterization and biological effects of Sicilian Opuntia ficus-indica (L.)mill. fruit juice: antioxidant and antiulcerogenic activity. J. Agric. Food Chem 2003; 51:4903–4908 6. Chaalal M, Louaileche H, Touati N, Bachir Bey M. Phytochemicals, in vitro antioxidant capacity and antiradical potential of whole and ground seeds of three prickly pear varieties: a comparative study. Ind. Crops Prod 2013; 49:386–391 7. Dok-Go H, Lee KH, Kim HJ, Lee EH, Lee J, Song YS, Lee Y-H, Jin C, LeeYS, Cho J. Neuroprotective effects of antioxidative flavonoids, quercetin,(+)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res 2003; 965:130–136 8. Alimi H, Hfaiedh N, Bouoni Z, Sakly M, Ben Rhouma K. Evaluation of antioxidant and antiulcerogenic activities of Opuntia ficus-indica f. inermis flowers extract in rats. Environ. Toxicol. Pharmacol 2011; 32:406–416 9. Kuti JO. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem 2004; 85 (4):527-533 10. Jordá FC, López Vivancos J: Fatigue as a determinant of health in patients with celiac disease. J Clin Gastroenterol 2010; 44:423-427 11. Fukuda K, Straus SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome: a comprehensive approach to its definition and study. International Chronic Fatigue Syndrome Study Group. Ann Intern Med 1994; 121(12):953-9 12. Adler RH. Chronic fatigue syndrome (cfs).Swiss Med Wkly 2004; 134 (19-20):268-76 13. Arcoleo A, Ruccia M, Cusmando S. Flavonoid pigments from Opuntia. I. Isorhamnetin from flowers Opuntia ficus-indica. Annal Chimica (Rome) 1961; 51:81 14. Graefe EU, Derendorf H, Veit M. Pharmacokinetics and bioavailability of the flavonol quercetin in humans. Int J Clin Pharmacol Ther 1999; 37(5):219-33 15. Olthof MR, Hollman PCH, Vree TB, Katan MB. Bioavailabilities of quercetin-3-glucoside and quercetin-4'-glucoside do not differ in humans. J Nutr 2000; 130(5):1200-1203 16. Rasmussen SE, Breinholt VM: Non-nutritive bioactive food constituents of plants: bioavailability of flavonoids. Int J Vitam Nutr Res 2003; 73(2):101-11 17. Park SH, Sim YB, Han PL, Lee JK, S HW. Antidepressant-like Effect of Kaempferol and Quercitirin, Isolated from Opuntia ficus-indica var. saboten. Exp Neurobiol. 2010; 19(1):30–38 18. Smets EMA, Garssen B, Bonke B, De Haes JCJM: The multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res 1995; 39(3):315-325 19. Sheehan DV, Harnett-Sheehan K, Raj BA: The measurement of disability. Int Clin Psychopharmacol 1996; 11 (Suppl 3):89-95 20. Leon AC, Olfson M, Portera L, Farber L, Sheehan DV: Assessing psychiatric impairment in primary care with the Sheehan Disability Scale. Int J Psychiatry Med 1997; 27(2):93-105 21. Bullinger M, Kirchberger I, Ware J: Der deutsche SF-36 Health Survey. Übersetzung und psychometrische Testung eines krankheitsübergreifenden Instruments zur Erfassung der gesundheitsbezogenen Lebensqualität. Zeitschrift für Gesundheitswissenschaften 1995; 3: 21-36 22. Fisk JD, Ritvo PG, Ross L, Haase DA, Marrie TJ, Schlech WF: Measuring the functional impact of fatigue: initial validation of the fatigue impact scale. Clin Infect Dis 1994; 18 (Suppl 1):79-83 23. Häuser W, Almouhtasseb R, Muthny FA, Grandt D: Validierung der deutschen Version der Fatigue Impact Scale FIS-D. Z Gastroenterol 2003; 41(10):973-982 24. Berk M, Ng F, Dodd S, Callaly T, Campbell S, Bernardo S, Trauer T: The validity of the CGI severity and improvement scales as measures of clinical effectiveness suitable for routine clinical use. J Eval Clin Pract 2008; 14(6):979-83 25. Kroke A, Klipstein-Grobusch K, Voss S, Möseneder J, Thielecke F, Noack R, Boeing H: Validation of a self-administered food-frequency questionnaire administered in the European Prospective Investigation into Cancer and Nutrition (EPIC) Study: comparison of energy, protein, and macronutrient intakes estimated with the doubly labeled water, urinary nitrogen, and repeated 24-h dietary recall methods. Am J Clin Nutr 1999; 70(4):439-47 26. Deutsche Gesellschaft für Ernährung, Österreichische Gesellschaft für Ernährung, Schweizerische Gesellschaft für Ernährungsforschung, Schweizerische Vereinigung für Ernährung (Ed). Referenzwerte für die Nährstoffzufuhr. 1. Auflage, 4. korrigierter Nachdruck. Neustadt a. d. Weinstraße: Neuer Umschau Buchverlag ; 2012. 27. Sharpe MC, Archard LC, Banatvala JE, Borysiewicz LK, Clare AW, David A, Edwards RH, Hawton KE, Lambert HP, Lane RJ, et al. A report - chronic fatigue syndrome: guidelines for research. J. R. Soc. Med 1991; 84(2):118-121 28. Weatherley-Jones E, Nicholl JP, Thomas KJ, Parry GJ, McKendrick MW, Green ST, Stanley PJ, Lynch SP. A randomised, controlled, triple-blind trial of the efficacy of homeopathic treatment for chronic fatigue syndrome. J Psychosom Res 2004; 56(2):189-97 29. Arnold LM, Clauw D, Wang F, Ahl J, Gaynor PJ, Wohlreich MM. Flexible dosed Duloxetine in the treatment of fibromyalgia: A randomized, double-blind, placebo-controlled trial. J Rheumatol 2010; 37:12 30. Schmidt D. Proof of principle trials: exploratory open studies Epilepsy Res 2001; 45:15–18. 31. Schmidt B. Proof of principle studies. Reviews / Epilepsy Res (2006); 68:19–94