Preclinical safety assessment and mutagenicity of the hydroethanolic extract of Syzygium campanulatum leaves.
Keywords:
Syzygium campanulatum, Myrtaceae, Mutagenicity, Acute toxicity, Subacute, Subchronic toxicityAbstract
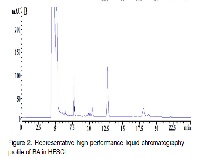
Syzygium campanulatum (Myrtaceae) is a species indigenous to Southeast Asia, a widely consumed medicinal herb and rich in phytochemical content and notable antiangiogenic and anti-colon cancer. Safety reports of administration of S. campanulatum are however lacking. In this study, we investigated the quality of dried leaves, chemical composition analyzed by FTIR and HPLC, phytochemicals content and repeated doses toxicity and mutagenicity effect of the hydroethanolic extract of S. campanulatum leaves (HESCL). The rats were divided into experimental and control groups and fed with 500, 1000, 2000 mg/kg/day of the hydroethanolic extract of S. campanulatum leaves for 28 and 90 days and with a single dose of 5000 mg/kg in acute study. The obtained results showed the dry leaves of S. campanulatum were devoid of any heavy metal and microbial contamination. The major components of HESCL were, respectively betulinic acid (60.43 mg/g.), total glycol saponins, total phenolics, total proteins, total tannins, and total flavonoids. No mutagenicity was detected in S. typhimurium auxotroph and no signs of clinical toxicity and mortality were observed in the experimental groups after 28 and 90-day experiment. However, significant (p < 0.05) statistical deviations were observed in hematological, and biochemical parameters but they were within the normal clinical range for rat, therefore not considered treatment-related. Based on these findings the fifty percent lethal dose was > 5000 mg/kg and the NOAEL was up to 2000 mg/kg for 90 days, as such HESCL is a relatively safe herb.
References
. Veiga Junior VF, Pinto AC, Maciel MAM. Medicinal plants: safe cure? Química Nova. 2005;28:519-28.
. Saad B, Azaizeh H, Abu-Hijleh G, Said O. Safety of traditional Arab herbal medicine. Evid Based Complement Alternat Med. 2006;3:433-9.
. Debelle FD, Vanherweghem J-L, Nortier JL. Aristolochic acid nephropathy: a worldwide problem. Kidney Int. 2008;74:158-69.
. Ifeoma O, Oluwakanyinsola S. Screening of Herbal Medicines for Potential Toxicities: INTECH Open Access Publisher; 2013.
. OECD. OECD Guidelines for the Testing of Chemicals. Paris: OECD Publishing; 2015.
. Gad SC. Preclinical development handbook: toxicology. New York City, US: John Wiley & Sons; 2008.
. Aisha A, Abu-Salah K, Darwis Y, Abdul Majid A. Screening of antiangiogenic activity of some tropical plants by rat aorta ring assay. Int J Pharmacol. 2009;5:370-6.
. Aisha AF, Ismail Z, Abu-Salah KM, Siddiqui JM, Ghafar G, Majid AMSA. Syzygium campanulatum korth methanolic extract inhibits angiogenesis and tumor growth in nude mice. BMC Complement Altern Med. 2013;13:168-79.
. Memon AH, Ismail Z, Aisha AF, Al-Suede FSR, Hamil MSR, Hashim S, et al. Isolation, Characterization, Crystal Structure Elucidation, and Anticancer Study of Dimethyl Cardamonin, Isolated from Syzygium campanulatum Korth. Evid Based Complement Alternat Med. 2014;2014:1-11.
. WHO. Evaluation of certain food additives and contaminants. World Health Organ Tech Rep Ser. Geneva, Switzerland: Joint FAO WHO Expert Committee on Food Additives; 2000. p. 1.
. USP USP. Method for the Microbiological Examination of Food. Washington, United State: United State Pharmacopoeia; 2009.
. Farsi E, Shafaei A, Hor SY, Ahamed MB, Yam MF, Asmawi MZ, et al. Genotoxicity and acute and subchronic toxicity studies of a standardized methanolic extract of Ficus deltoidea leaves. Clinics (Sao Paulo). 2013;68:865-75.
. Ames BN, McCann J, Yamasaki E. Methods for detecting carcinogens and mutagens with the Salmonella/mammalian-microsome mutagenicity test. Mutat Res Genet Toxicol Environ Mutagen. 1975;31:347-63.
. OECD. Test No. 423: Acute Oral toxicity - Acute Toxic Class Method. Paris: OECD Publishing; 2002.
. OECD. Test No. 425: Acute Oral Toxicity: OECD Guidelines for the Testing of Chemicals. In: Chemicals OGftTo, editor. Paris: OECD Publishing; 2008.
. OECD. Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents, OECD Guidelines for the Testing of Chemicals. Paris: OECD Publishing; 1998.
. Bernal J, Mendiola JA, Ibáñez E, Cifuentes A. Advanced analysis of nutraceuticals. Journal of Pharmaceutical and Biomedical Analysis. 2011;55:758-74.
. Zhou Q, Sun S-Q, Zuo L. Study on traditional Chinese medicine ‘Qing Kai Ling’ injections from different manufactures by 2D IR correlation spectroscopy. Vib Spectrosc. 2004;36:207-12.
. Hayes AW, Kruger CL. Hayes' principles and methods of toxicology. Boca Raton, US: CRC Press; 2014.
. Hodge HC, Sterner JH. Tabulation of toxicity classes. American Industrial Hygiene Association Quarterly. 1949;10:93-6.
. Evans GO. Animal Hematotoxicology: A Practical Guide for Toxicologists and Biomedical Researchers. Abingdon, US: CRC Press; 2008.
. Giknis ML, Clifford CB. Clinical Laboratory Parameters for Crl CD(SD) Rats. USA: Charles River Laboratories International, Inc.; 2006.
. Evans GO. Animal Clinical Chemistry: A Practical Handbook for Toxicologists and Biomedical Researchers. Second ed. Abingdon, US: CRC Press; 2009.
. Hamdy RC, Lewiecki EM. Osteoporosis. Northamptonshire, US: Oxford University Press; 2013.
. Naber TH, Baadenhuysen H, Jansen JB, van den Hamer CJ, van den Broek W. Serum alkaline phosphatase activity during zinc deficiency and long-term inflammatory stress. Clin Chim Acta. 1996;249:109-27.
. Cho YE, Lomeda RAR, Ryu SH, Sohn HY, Shin HI, Beattie JH, et al. Zinc deficiency negatively affects alkaline phosphatase and the concentration of Ca, Mg and P in rats. Nutr Res Pract. 2007;1:113-9.
. Baker HJ, Lindsey JR, Wesibroth SH. The Laboratory Rat: Biology and Diseases: Elsevier Science; 2013.
. Zimmerman HJ. Hepatotoxicity: The Adverse Effects of Drugs and Other Chemicals on the Liver. Philadelphia, USA: Lippincott Williams & Wilkins; 1999.
. Sharp P, Villano JS. The Laboratory Rat, Second Edition: Taylor & Francis; 2012.
. Hor SY, Ahmad M, Farsi E, Yam MF, Hashim MA, Lim CP, et al. Safety assessment of methanol extract of red dragon fruit (Hylocereus polyrhizus): Acute and subchronic toxicity studies. Regul Toxicol Pharmacol. 2012;63:106-14.