Ameliorative Effect of Chlorophyllin on Oxidative Stress in Experimental Model of Diabetes
Keywords:
Diabetes, Oxidative stress, Streptozotocin, Chlorophyllin, Ascorbic acidAbstract
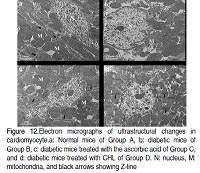
The aim of this present study was to investigate the effect of chlorophyllin (CHL) on oxidative stress in Streptozotocine (STZ) induced diabetic mice. For the study, mice were divided into Group A: normal control, Group B: diabetic control, Group C: diabetic mice treated with the ascorbic acid, and Group D: diabetic mice treated with CHL. Levels of Reactive Oxygen Species (ROS), lipid peroxidation, protein carbonyl, superoxide dismutase (CuZn SOD &Mn-SOD), catalase (CAT), glutathione peroxidase (GPx), and glutathione reductase (GR) activities were examined in kidney and heart tissues of different experimental groups. Histological and ultrastructuralstudies were also carried out to evaluate any changes in tissues as well as sub-cellular organelles. ROS, lipid peroxidation, and protein carbonyl levels have been significantly decreased with concomitant increased of CuZn SOD, Mn-SOD, CAT, GPx, and GR activity in CHLtreated diabetic mice. The histological and ultrastructural studies showed that CHL attenuates the detrimental effect of oxidative stress and alleviated tissue injuries in STZ induced diabetic mice. These results suggested that CHL possesses antioxidative activity and has the potential to amelioratediabetes-associated oxidative stress in mice.
References
. Inoguchi T, Sonta T, Tsubouchi H, Etoh T, Kakimoto M, Sonoda N, Sato N, Sekiguchi N, Kobayashi K, Sumimoto H, Utsumi H, Nawata H. Protein Kinase C-dependent increase in ROS production in vascular tissues of diabetes: role of vascular NAD(P)H Oxidase. J Am Soc Nephrol 2003,14(8 Suppl 3):227–232.
. Williamson JR, Chang K, Frangos M, Hasan KS, Ido Y, Kawamura T, Nyengaard JR, Enden MVD, Kilo C, Tilton RG. Perspectives in diabetes. hyperglycemic pseudohypoxia and diabetic complications. Diabetes 1993,42(6):801–813.
. Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes 1999,48(1):1–9.
. Ceriello A. Oxidative stress and glycemic regulation. Metabolism 2000,49(2 Suppl 1):27–29.
. Chang CLT, Lin Y, Bartolome AP, Chen YC, Chiu SC, Yang WC. Herbal therapies of Type 2 DM: chemistry, biology, and potential application of selected plants and compounds. Evid Based Complement Alternat Med 2013;2013:378657.
. Saio V, Syiem D, Sharma S, Dkhar J. Amelioration of age-dependent increase in oxidative stress markers in male mice by extract of Potentilla fulgens. Redox Rep 2016;21(3):130-138.
. Dashwood R, Negishi T, Hayatsu H, Breinholt V, Hendricks J, Bailey G. Chemopreventive properties of chlorophylls towards aflatoxin B1: a review of the antimutagenicity and anticarcinogenicity data in rainbow trout. Mutat Res 1998;399(2):245–253.
. Kumar SS, Devasagayam TP, Bhushan B, Verma NC. Scavenging of ROS by CHL: an ESR study. Free Radic Res 2001;35(5):563-574.
. Patar AK, Bhan S, Syiem D. Effect of CHL , an semi-synthetic chlorophyll molecule on hyperglycemia and hyperlipidemia in STZ-induced diabetic mice. Int J Pharm Pharm Sci 2016;8(8):293-296.
. Sharma S, Choudhary M, Bhardwaj S, Choudhary N, Rana AC.Hypoglycemic potential of alcoholic root extract of Cassia occidentalis Linn. in STZ-induced diabetes in mice. B-FOPCU 2014;52(2):211-217.
. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 1976;254(1-2):248–254.
. LeBel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe DCF as an indicator of ROS formation and oxidative stress. Chem Res Toxicol 1992;5(2):227–231.
. Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 1979;95(2):351–358.
. Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 1990;186:464–478.
. Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for SOD. Eur J Biochem 1974;47(3):469–474.
. Aebi H. CAT in vitro. In: Methods Enzymol. New York, USA: Academic Press; 1984. p.121–6.
. Paglia DE, Valentine WN. Studies on the quantitative and qualitative characterization of erythrocyte GPx. J Lab Clin Med 1967;70(1):158–169.
. Lawrence RA, Burk RF. GPx activity in selenium-deficient rat liver. Biochem Biophys Res Commun 1976; 71(4):952–958
. Carlberg I, Mannervik B. GR. Methods Enzymol 1985;113:484–490.
. Kiernan JA. Histological and histochemical methods:theory and practice, 3rd ed. Oxford, England: Butterworth–Heinemann Publishers; 1999.
. Hayat MA. Basic techniques for transmission electron microscopy. New York, USA: Academic Press; 1985.
. Noberasco G, Odetti P, Boeri D, Maiello M, Adezati L. MDA level in diabetic subjects. relationship with blood glucose and glycosylated hemoglobin. Biomed Pharmacother 1991;45(4-5):193–196.
. Pandey KB, Mishra N, Rizvi SI. Protein oxidation biomarkers in plasma of type 2 diabetic patients. Clin Biochem 2010;43(4-5):508–511.
. Suzuki D, Miyata T. Carbonyl stress in the pathogenesis of diabetic nephropathy. Intern Med 1999;38(4):309–314.
. Nystrom T. Role of oxidative carbonylation in protein quality control and senescence. Embo J 2005;24(7):1311–1317.