Anti-Helicobacter pylori effect of the antioxidant extract from Baccharis trimera Less. (DC)
Keywords:
antioxidant, morphological alterations, secondary metabolites, Helicobacter pyloriAbstract
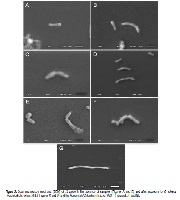
One of the main risk factors for the development of stomach ulcers and cancer is infection with Helicobacter pylori bacteria, which is accompanied by considerable oxidative stress. Therefore, the aim of the present study was to assess the anti-Helicobacter pylori activity of B. trimera hydroalcoholic extract (HE) and aqueous (AqF), hexanic (HxF), and acetonitrile/chloroform (ACF) fractions, as well as their oxidant potentials. A preliminary phytochemical screening was carried out. Anti-Helicobacter pylori activity was assessed using a microdilution assay. After exposure to the investigated samples, the bacterial morphology was analysed under a scanning electron microscope (SEM). The antioxidant activity was evaluated in hydrogen peroxide (H 2 O 2 ), superoxide anion (O 2 •- ), hypochlorous acid (HOCl), hydroxyl radical (HO • ) and nitric oxide (NO • ) assays. The highest concentration of polyphenols was found in HE, the highest concentration of flavonoids was found in ACF, and of tannins was found in AqF. In the anti-H. pylori assay, the MIC 90 was 512 µg/mL for HE and 1024 µg/mL for ACF, which was bactericidal. The SEM showed morphological alterations such as cell lysis in the tested samples. In the O 2 •- inhibition assay, the EC 50 of AqF was 5.85 ± 0.86. In the HOCl, HO • radical, NO • and H 2 O 2 scavenging assays, the best results were in ACF, with an EC 50 of 15.50 ± 0.80, 2.90 ± 0.48, 132.13 ± 7.38 and 66.70 ± 2.30 µg/mL, respectively. The analyses indicate that compounds present in B. trimera, especially in HE and ACF, are promising candidates for the prevention and treatment of diseases caused by H. pylori.
References
. Vítor JMB, Vale FF. Alternative
therapies for Helicobacter pylori:
probiotics and phytomedicine.
FEMS Immunol Med Microbiol.
;63:153-164.
. Sugano K, Tack J, Kuipers EJ, Graham
DY, El-Omar EM, Miura S, Hamura K,
Asaka M, Uemura N, Malfertheiner P.
Kyoto global consensus report on
Helicobacter pylori gastritis. Gut.
;0:1-15.
. Polk DB, Peek RM. Helicobacter pylori:
gastric cancer and beyond. Nat Rev
Cancer. 2010;10:403-414.
. Marshall BJ, Warren JR. Unidentified
curved bacilli in the stomach of patients
with gastritis and peptic ulceration.
Lancet. 1984;1:1311-1314.
. Ladeira MSP, Salvadori DMF,
Rodrigues MAM. Biopathology of
Helicobacter pylori. J Bras Patol Med
Lab. 2003;39:335-342.
. Bhattacharyya A, Chattopadhyay R,
Mitra S, Crowe SE. Oxidative stress:
an essential factor in the pathogenesis
of gastrointestinal mucosal diseases.
Physiol Rev. 2014;94:329-354.
. Verdi LG, Brighente IMC, Pizzolatti
MG. Gênero Baccharis (Asteraceae):
Aspectos químicos, econômicos e
biológicos. Quim Nova. 2005;28:85-94.
. Abad MJ, Bermejo P. Baccharis
(Compositae): a review update.
ARKIVOC. 2007;7:76-96.
. Campos FR, Bressan J, Jasinski VCG,
Zuccolotto T, Silva LE da, Cerqueira
LB. Baccharis (Asteraceae): Chemical
Constituents and Biological Activities.
Chem Biodivers. 2016;13:1-17.
. Simões-Pires CA, Queiroz EF,
Henriques AT, Hostettmann K.
Isolation and on-line identification of
anti- oxidant compounds from three
Baccharis species by HPLC-UV-MS /
MS with post-column derivatisation.
Phytochem Anal. 2005;16:307-314.
. Oliveira CB, Comunello LN, Lunardelli
A, Amaral RH, Pires MGS, da Silva GL,
Manfredini V, Vargas CR, Gnoatto
SCB, Oliveira JR, Gosmann G.
Phenolic enriched extract of Baccharis
trimera presents anti-inflammatory and
antioxidant activities. Molecules.
;17:1113-1123.
. BRASIL. RENISUS - Relação nacional
de plantas medicinais de interesse ao
SUS. Ministério da Saúde. 2009.
Disponível em:
http://portalsaude.saude.gov.br/images
/pdf/2014/maio/07/renisus.pdf. Acesso
em: 12 fev. 2016.
. BRASIL. Agência Nacional de
Vigilância Sanitária. Resolução de
Diretoria Colegiada (RDC) nº 26 de
05.2014. Dispõe sobre o registro de
medicamentos fitoterápicos e o registro
e a notificação de produtos tradicionais
fitoterápicos. Ministério da Saúde.
. Neves LC, Alencar SM de, Carpes ST.
Determinação da atividade
antioxidante e do teor de compostos
fenólicos e flavonoides totais em
amostras de pólen apícola de Apis
mellifera. Braz J Food Technol.
;107-110.
. Dimech GS, Soares LAL, Ferreira MA,
Oliveira AGV de, Carvalho M da C,
Ximenes EA. Phytochemical and
antibacterial investigations of the
extracts and fractions from the stem
bark of Hymenaea stigonocarpa Mart .
ex Hayne and effect on ultrastructure of
Staphylococcus aureus induced by
hydroalcoholic extract. Sci World J.
;2013:1-8.
. Perdigão TL: Avaliação
morfofisiológica, fitoquímica e
mutagênica de Passiflora edulis Sims f.
flavicarpa Derg exposta a diferentes
concentrações de alumínio.
Dissertação de Mestrado. Universidade
Federal do Espírito Santo, Centro de
Ciências Humanas e Naturais; 2012.
. Clinical and Laboratory Standards
Institute (CLSI): Methods for dilution
antimicrobial susceptibility tests for
bacteria that grow aerobically. CLSI
document M07- A6. 2003;23:1-49.
. Tanaka T, Kawase M, Tani S. Urease
inhibitory activity of simple α,β-
unsaturated ketones. Life Sci.
;73:2985-2990.
. Ching T-L, Jong J, Bast A. A method
for screening hypochlorous acid
scavengers by inhibition of the
oxidation of 5-Thio-2-Nitrobenzoic Acid:
Application to anti-asthmatic drugs.
Anal Biochem. 1994;218:377-381.
. Suzumura K, Yasuhara M, Narita H.
Superoxide anion sacavenging
properties of fluvastatin and its
metabolites. Chem Pharm Bull.
;47:1477-1480.
. [Pick E, Keisari Y. A simple colorimetric
method for the measurement of
hydrogen peroxide produced by cells in
culture. J Immunol Methods.
;38:161-170.
. Halliwell B, Gutteridge JMC, Aruoma
OI. The deoxyribose method: A simple
“test-tube” assay for determination of
rate constants for reactions of hydroxyl
radicals. Anal Biochem. 1987;165:215-
. Marcocci L, Maguire JJ, Droy-Lefaix
MT, Packer L. The nitric oxidescavenging properties of ginkgo biloba
extract EGb 761. Biochem Biophys
Res Commun. 1994;201:748-755.
. Barkun A, Leontiadis G. Systematic
review of the symptom burden, quality
of life impairment and costs associated
with peptic ulcer disease. Am J Med.
;123:358-366.
. Ding S-Z, Minohara Y, Fan XJ, Wang
J, Reyes VE, Patel J, Dirden-Kramer B,
Boldogh I, Ernst PB, Crowe SE.
Helicobacter pylori infection induces
oxidative stress and programmed cell
death in human gastric epithelial cells.
Infect Immun. 2007;75:4030-4039.
. Curtis NAC, Orr D, Ross GW, Boulton
MG. Competition of β-Lactam
antibiotics for the penicillin-binding
proteins of Pseudomonas aeroginosa,
Enterobacter cloacae, Klebsiella
aerogenes, Proteus rettgeri, and
Escherichia coli: Comparasion with
antibacterial activity and effects upon
bacterial morphology. Antimicrob
Agents Chemother. 1979;16:325-328.
. DeLoney CR, Schiller NL. Competition
of various beta-lactam antibiotics for
the major penicillin-binding proteins of
Helicobacter pylori: antibacterial activity
and effects on bacterial morphology.
Antimicrob Agents Chemother.
;43:2702-2709.
. Dzoyem JP, Hamamoto H, Ngameni B,
Ngadjui BT, Sekimizu K. Antimicrobial
action mechanism of flavonoids from
Dorstenia species. Drug Discov Ther.
;7:66-72.
. Jasmine R, Selvakumar BN, Daisy P.
Investigating the mechanism of action
of terpenoids and the effect of
interfering substances on an indian
medicinal plant extract demonstrationg
antibacterial activity. Int J Pharm Stud
Res. 2011;2:19-24.
. Shahidi F, Ambigaipalan P. Phenolics
and polyphenolics in foods, beverages
and spices: Antioxidant activity and
health effects - A review. J Funct
Foods. 2015;18:820-897.
. Wang Y, Wang X. Binding, stability,
and antioxidant activity of quercetin
with soy protein isolate particles. Food
Chem. 2015;188:24-29.
. Gülçin İ, Huyut Z, Elmastaş M, AboulEnein HY. Radical scavenging and
antioxidant activity of tannic acid. Arab
J Chem. 2010;3:43-53.
. Vieira TO, Seifriz I, Charão CCT,
Oliveira SQ, Creczynski-Pasa TB.
Antioxidant effects of crude extracts
from Baccharis species: inhibition of
myeloperoxidase activity, protection
against lipid peroxidation, and action as
oxidative species scavenger. Rev Bras
Farmacogn. 2011;21:601-607.
. Khan MS, Priyadarshini M, Bano B.
Preventive effect of curcumin and
quercetin against nitric oxide mediated
modification of goat lung cystatin. J
Agric Food Chem. 2009;57:6055-6059.
. Bendary E, Francis RR, Ali HMG,
Sarwat MI, El Hady S. Antioxidant and
structure-activity relationships (SARs)
of some phenolic and anilines
compounds. Ann Agric Sci.
;58:173-181.