Anti-androgenic, anti-oestrogenic and antioxidant activities of aqueous extract of Laportea ovalifolia on adults rats.
Keywords:
Laportea ovalifolia, prostate cancer, anti-androgenic activity, anti-estrogenic, antioxidant activityAbstract
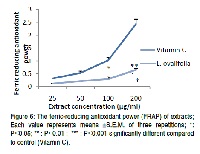
Cancer is one of the most life-threatening diseases in which deregulating proliferation of abnormal cells invades and disrupts surrounding tissues. It constitutes seriouspublic health problems in both developed and developing countries. To evaluate the anti-androgenic, anti-estrogenic and antioxidant activities of Laportea ovalifolia (L. ovalifolia) in order to contribute to the search and the valorization of medicinal plants which could reduce mortality related to prostate cancer. The evaluation of the anti-androgenic activity were carried out on castrated male rats receiving simultaneous daily administration of testosterone and different doses of aqueous extract of L. Ovalifolia during a period of 10 days. That of the anti-estrogenic activity was carried out on mature ovariectomized female rats receiving for a week simultaneous daily administration of estradiol and different doses of plant extract. The evaluation of the in vivo antioxidant activity of L. Ovalifolia aqueous extract was carried on adult male rats receiving simultaneous daily administration of naphthalene and different doses of extract, for 15 days. For its in vitro antioxidant activity, the amounts of phenolic compounds in plant extracts were determined as well as the total flavonoid contents of the crude extracts. Also, the DPPH scavenging activity of the plant extract was determined as well as its reducing power. As compare to the 0 mg/kg testosterone primed castrated rat, those treated with the various dose of the plant extract presented either a significant decrease in weights of all their reproductive tissues (P˂0.01 - P˂0.001) or a significant increase (P˂0.001) in their serum level of testosterone. For all the plant extract treated ovariectomized rats, similar trends were observed for the relative uteri weight (P˂0.01) and that of the serum level of estradiol (P˂0.001). Plant extract contains 13.33±0.1 mg GAE/g and 05.27±0.17 mg CATE/g of phenolic and flavonoids compounds respectively and exhibits DPPH radical scavenging ability as well as ferric-reducing antioxidant power. Relatively to animals treated at 0 mg/kg, the various doses of the plant extract significantly increased (P˂0.05 - P˂0.001) the activity of catalase (in liver, lungs and the serum), SOD (in liver and heart) and peroxidase (in liver, heart, serum and lungs). It also significantly reduces (P˂0.001) the level of nitric oxide in the liver, heart, lungs, kidneys and serum. Globally, these results denote the anti-androgenic, anti-estrogenic and antioxidant potential of L. ovalifolia.
References
Mahamuni SS, Killedar SG, More HN, Kakade HR, Gadakari SS, Desai RB. Phytochemical screening of Lagenaria siceraria standely fruit and its anticancer activity using potato discs assay model. Int J Biol Pharm Allied Sci 2012; 1(5): 675-683.
Sies H, Stahl W, Sundquist AR. Antioxidant functions of vitamins. Vitamins E and C, beta-carotene, and other carotenoids. Ann N Y Acad Sci 1992; 669:7-20.
Lim YY, Murtijaya J. Antioxidant properties of Phyllantusamurus extract as affected by different drying method. L W T 2007; 40:1664-1669.
Huggins C, Hodges CV. Studies on prostatic cancer. The effect of castration, of estrogen and of androgen interaction on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research 1941; 1 293–297.
Nelles JL, Hu WY, Prins GS. Estrogen Action and Prostate Cancer. Expert Rev Endocrinol Metab 2011; 6(3):437-451.
Ellem SJ and Risbridger GP. Treating prostate cancer: a rationale for targeting local oestrogens. Nature Reviews Cancer 2007; 7 621–627. ¬
Tagne R S, Telefo B P, Njina S N, Bala B, Goka S C, Yemele D M et al. In vivo anti-androgenic, anti-estrogenic and antioxidant activities of the aqueous extract of Eremomastax speciosa, Asian Pac J Trop Dis 2014a; 4(Suppl 2): S952-S956.
Tagne R S, Telefo B P, Nyemb J N, Yemele D M, Njina S N, Goka S C, et al. Anticancer and antioxidant activities of methanol extracts and fractions of some Cameroonian medicinal plants. Asian Pac J Trop Med 2014b; 7(Suppl 1): S442-S447.
Kim IY, Seong do H, Kim BC, Lee DK, Ramaley AT, Leach F et al. Raloxifene, a selective estrogen receptor modulator, induces apoptosis in androgen-irresponsive human prostate cancer cell line LNCaP through an androgen-independent pathway. Cancer Res 2002; (13) 3649-3653.
EEC, 1986.CouncilDirective86/609/EE Cof 24 November1986 on the approximation of laws, regulations and administrative provisions of the Member States regarding the protection of animals used for experimental and other scientific purposes. Official Journal of the European Community, L358:1–29.
Spano GA, Wrolstad RE. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. Journal of Agricultural & Food Chemistry1990; (38): 1565–1571.
Lister E, Wilson. Measurement of total phenolics and ABTS assay for antioxidant activity (personal communication). Crop Research Institute 2001, Lincoln, New Zealand.
Lamaison JLC, Carnet A. Teneurs en principaux flavonoids des fleurs de Crataegeusmonogyna Jacq et de Crataegeuslaevigata (Poiret D. C) en function de la vegetation. Pharm Acta Helv 1990; (65): 315-320.
Gyamfi MA, Yonamine M, Aniya Y. Free radical scavenging activity of medicinal herb of Ghana: Thonningiasanguinea on experimentally induced liver injuries. General Pharmacol1999; (32):661-667.
Gow-CY and Hui-YC. Antioxidant Activity of Various Tea Extracts in Relation to Their Antimutagenicity. J Agric Food Chem 1995; (43), 27-32.
Pavlović SZ, Borković MSS, Radovanović TB, Perendija BR, Despotović SG, Gavrić JP, et al. Seasonal variations of the activity of antioxidant defense enzymes in the red mullet (Mullusbarbatus l.) from the Adriatic Sea. Mar Drugs 2010; 8(3): 413-428.
Habbu PV, Shastry RA, Mahadevan K M, Hanumanthachar J, Das SK. Hepatoprotective And Antioxidant Effects of Argyreia Speciosa In Rats. Afr. J. Trad. CAM 2008; 5 (2): 158 -164
Wilson VS, Blystone CR, Hotchkiss AK, Rider CV, Gray LE. Diverse mechanisms of anti-androgen action: impact on male rat reproductive tract development. Int J Androl 2008; 31(2): 178-187.
De Oliveira DH, Fighera TM, Bianchet LC, Kulak CA, Kulak J (2012). Androgens and bone. Minerva Endocrinol; 37(4): 305-314.
Basly JP, Lavier MC. Dietary phytoestrogens : potential selective estrogen enzyme modulators? Planta Med 2005 (71): 287-294.
Adlercreutz H (). Phyto-oestrogens and cancer. Lancet Oncol 2002; (3): 364-373.
Pihlajamaa P, Zhang F, Saarinen L, Mikkonen L, Hautaniemi S. The phytoestrogen genistein is a tissue-specific androgen receptor modulator. Endocrinology 2011; (152): 4395-4405.
Leon H, Bradlow HL , Freed S, Levin J, Rosenfeld RS ,Whitmore WF et al. The Effect of Flutamide on Testosterone Metabolism and the Plasma Levels of Androgens and Gonadotropins.DOI: http://dx.doi.org/10.1210/jcem-45-6-1224, 2013
Yildiz F. Phytoestrogens in Functional Foods, CRC Boca Raton FL 2005.
Oben JE, Assi SE, Agbor GA, Musoro DF. Effect of Eremomastax speciosa on experimental diarrhea. Afr J Tradit Complenet Altern Med 2006; (3): 95-100. ¬
Gustafsson Jk.Therapeutic potential of selective estrogen receptor modulators. Current Opinion in Chemical Biology 1998; 2(4):508–511.
Hong WK, Sporn MB. Recent Advances in Chemoprevention of Cancer. Science 1997; 278(5340):1073-1077.
Piccolella M, Valeria C, Elio M, Marc J, Angelo P. Modulators of estrogen receptor inhibit proliferation and migration of prostate cancer cells. Pharmacological Research 2014; 79 (2014) 13– 20
Steiner MS, Raghow S. Antiestrogens and selective estrogen receptor modulators reduce prostate cancer risk. World Journal of Urology 2003; 21(1):31-36.
Brigitte U, Francois H, Imre, Michael WD, and Rene R. Raloxifene Treatment Is Associated With Increased Serum Estradiol and Decreased Bone Remodeling in Healthy Middle-Aged Men With Low Sex Hormone Levels. J Bone Miner Res 2002; (19):1518 –1524.
Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trend Plant Sci1997; (4):152-159.
Willems BAT, Melnick RL, Kohn MC, and Portier CJ. A physiological based pharmacokinetic model for inhalation and intravenous administration of naphthalene in rats and mice. Toxicol Appl Pharmacol 2001; 176: 81-91.
Stohs SJ, Ohia S and Bagchi D. Naphthalene toxicity and antioxidant nutrients. Toxicol 2002;180: 97-105.
Tingle MD, Primohamed M, Templeton E, Wilson AS, Madden S, Kitteringham NR et al. An investigation of the formation of cytotoxic, genotoxic, protein-reactive and stable metabolites from naphthalene by human liver microsomes. Biochem Pharmacol1993; 46: 1529-1538.
Shi H, Yunxia S, Wang X, Luo Y, and Ji L. Hydroxyl radical production and oxidative damage induced by cadmium and naphthalene in liver of Carassiusauratus. Comp Biochem Physiol C 2005; 140: 115-121.
Beligni M, and Lamattina L. Is nitric oxide toxic or protective? Trends in Plant Science 1999; 4, 299-300.
Hawkins RD. NO honey, I don't remember. Neuron 1996; (16) 465-467.