The Protective Effects of Curcuminand Caffeic acid alone or in combination onNicotine-induced Lung Injury in Rats
Keywords:
Nicotine, lung, curcumin, caffeic acid, N-acetylcysteine, oxidative stress biomarkersAbstract
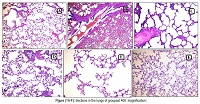
The present study was performed to explore the protective effects ofcaffeic acid (20 mg/kg.bw) and curcumin (50mg/k.g.b.w.) on nicotine-induced lung injury alone and in combination.Their effect was compared to N-acetylcysteine (500mg/k.g.b.w.) as known modulator of oxidative stress. Nicotine treatment (0.6mg/kg/day, i.p, for 21 consecutivedays) resulted in a significantincrease (p<0.05) in plasmaalanine aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), total cholesterol (TC), triglycerides (TG), low density lipoprotein cholesterol (LDL-C) and well as plasma and lung thiobarbituric acid reactive substances (TBARS), nitric oxide(NO) and tumor necroses factor-α (TNF-α) concomitant with significant decline in non-enzymatic antioxidant like reduced glutathione(GSH) and in enzymatic antioxidants like catalase (CAT) andsuperoxide dismutase (SOD) as well as high density lipoprotein cholesterol (HDL-C). Furthermore, nicotine treatment caused severe injury indicated bythe histopathological examination of lung tissue compared to normal control group. Oral treatment with caffeic acid alone orcurcuminalone or in combination as well as N-acetylcysteine alone prevented the elevation in plasma ALT, AST, LDH,TC, TG, LDL-C, NO, TNF-α and TBARSlevels concomitant with an increments in the HDL-C, reduced glutathione GSH and antioxidant enzymes (CAT and SOD) and amelioration inhistopathological changes and injury induced by nicotine. Lung protection was prominent in curcuminand N-acetylcysteine alone more than caffeic acid alone or caffeic acid and curcuminin combination. Moreover, curcumin has the potential to be used in a combination therapy with caffeic acid, with decreasing the therapeutic dose of caffeic acid and therefore its side-effects.
References
Sener, G., Toklu, HZ.,Cetinel, S. β-Glucan protects against chronic nicotine induced oxidative damage in rat kidney and bladder. Environmental Toxicology Pharmacology, 2007; 23: 25–32.
Siktar, E., Ekinci, D., Siktar, E. Protective role of L-carnitine supplementation against exhaustive exercise induced oxidative stress in rats. European Journal of Pharmacology, 2011; 668(3): 407–413.
Nallella, A., Allamaneni, SS., Said, TM. Role of antioxidants in treatment of male infertility Agarwal: An overview of the literature. Reprod Biomed Online, 2004; 8:616–27.
Gitto E., Reiter, RJ.,Karbownik, M. Causes of oxidative stress in the pre- and perinatal period. Biology of the Neonate, 2002; 81: 146–157
Osuna, C., Reiter, R., Garcia, JJ. Inhibitory effect of melatonin on homocysteine-induced lipid peroxidation in rat brain homogenate. Pharmacology and Toxicology, 2002; 90: 32–37.
Halliwell, B., Gutteridge, JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochemical Journal, 1984; 219: 1–14.
Sharma OP. Antioxidant activity of curcumin and related compounds. Biochem. Pharmacol., 1979; 25, 2811-1812.
Toda S, Miyase T, Arichi H, Tanizawa H, Takino Y. Natural antioxidants III, antioxidant compounds isolated from rhizomes of curcuma Longa. Chem. Pharm. Bull., 1985; 33, 1725-1728.
Masuda T, Isobe J, Jitoe A, Nakatani N. Antioxidative curcuminoids from rhizomes of curcuma xanthorrhiza. Phytochemistry, 1992; 31, 3645-3647.
Masuda T, Hidaha K, Shinohara A, Mackawa T, Takeda Y, Yamaguchi H. Chemical studies on antioxidant mechanism of curcuminoid: analysis of radical reaction products from curcumin. J. Agric. Food. Chem., 1999; 47, 71-77.
Nadakarni AK (1954). Indian Materia Medica, Popular Book Depot, Bombay, 1st ed., pp.414-42.
Joe B, Vijaykumar M, Lokesh B. Biological properties of curcumin-cellular and molecular mechanisms of action. Crit. Rev. Food Sci. Natur., 2004; 44, 97-111.
Sahu SC, Washington MC. Effect of ascorbic acid and curcumin on quercetin-induced nuclear DNA damage, lipid peroxidation and protein degradation. Cancer Lett., 1992; 63, 237-241.
Lim G, Chu T, Young F. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J. Neurosci., 2001; 21, 8370-8377.
Kawamori T, Lubet R, Steele V. Chemopreventive effect of curcumin, a naturally occurring anti-inflammatory agent, during the promotion/progression stages of colon cancer. Cancer Res., 1999; 59, 597-601.
Challis, L, Beutler E.1987. Active transport of glutathionedisulfide from erythrocytes. In: Larson, A., Orrenius,S., Holmgren, A., Mannerwik, B., editors. Functionsof glutathione, biochemical, physiological,toxicological and clinical aspects, New York, USA,Raven Press, pp.65.
Chan J.H., Ho C.T. Antioxidant activities ofcaffeic acid and its related hydroxy cinnamic acidcompounds. J. Agric. Food Chem. 1997; 45, 2374-2378.
Chen Y.J., Shiao M.S., Wang S.Y. Theantioxidant caffeic acid phenethyl ester inducesapoptosis associated with selective scavenging ofhydrogen peroxide in human leukemic HL-60 cells.Anticancer Drugs 2001; 12, 143-149.
Lee K.J., Choi J.H., Khanal T., Hwang Y.P., Chung Y.C., Jeong H.G. Protective effect of caffeicacid phenethyl ester against carbon tetrachlorideinduced hepatotoxicity in mice. Toxicology. 2008; 248, 18-24.
Albini A, Morini M, Agostini FD, Ferrari N, Campelli F, Arena G, Noonan DM, Pesce C, Flora SD.Inhibition of angiogenesis driven Kaposi's, sarcoma tumor growth in nude mice by oral N-acetylcysteine. Cancer Res,2001; 61: 8171–8178.
Filho GP, Ferriera C, Schwengber A, Marroni C, Zettler C, Marroni N. Role of Nacetylcysteine on fibrosis and oxidative stress in cirrhotic rats. Arq. Gastroenteral, 2008; 45: 156–162.
Eskiocak S., Altaner S, Bayir S, Cakir E. The effect of N-acetylcysteine on brain tissue of rats fed with high cholesterol diet. Turk. J. Biochem, 2008; 33:58–63.
Heyman SN, Goldfarb M, Shina A, Karmeli F, Rosen S. N-acetylcysteine ameliorates renal microcirculation: studies in rats. Kidney Int, 2003; 63: 634–641.
Modi M, Kaul RK, Kannan GM, Flora SJS. Co-administration of zinc and Nacetylcysteine prevents arsenic induced tissue oxidative stress in male rats. J. Trace Elem. Med. Biol, 2006; 20: 197–204.
De Vries N, De Flora S. N-acetylcysteine . J. cell. Biochem. (suppl.), 1993; 17F: 270 – 277.
Varadharajan V, Ganesan J.Restoration of Antioxidant Activity by N-acetylcysteine and Gallic Acid on Kidney Tissue of Mercuric Chloride Intoxicated Wistar Rats. International Journal of Biological & Pharmaceutical Research, 2013; 4(4): 302-307.
Liu, RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. The Journal of Nutrition, 2004; 134: 3479S-3485S.
Miltonprabu S, Shagirtha. K.Caffeic acid potentially attenuates cadmium induced oxidative stress mediated hepatotoxicity in rats.The Journal of Free Radicals and Antioxidants. 2013; 139: 141 -1 52.
Choudhary D, Chandra D, Kale R. Modulation of radioresponse of glyoxalase system by curcumin. Ethanopharmacol., 1999; 64, 1-7.
Kucukardali Y, Cinan U, Acar HV, Ozkan S, Top C, Nalbant S, Cermik H, Cankir Z, Danaci M. Comparison of the C.L. Sprague, A.A. Therapeutic efficacy of 4-methylpyrazole and N-acetylcysteine on acetaminophen (paracetamol) hepatotoxicity in rats. Curr. Med. Res. Opin, 2005; 18: 78–81.
Chanarin, I. 1989. Text book of Laboratory Haematology: An Account of Laboratory techniques, Churchill Livingstone, New York PP. 107.
Marklund, S., Marklund, D. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem., 1974; 47:469.
Sinha, AK. Colorimetric assay of catalase. J. Anal Biochem. 1972; 47 (2): 389-94.
Reitman, S, Frankel, SA. Colorimetric method for the determination of serum oxaloacetic acid and glutamic pyruvic transaminases. Am. j. Clin. Pathol. 1957; 28: 56 – 63.
Buhl, SN, Jackson, K. Optimal conditions and comparison of lactate dehydrogenase catalysis of the lactate to pyruvate to lactate reactions in human serum at 25, 30 and 37 0C. Clin. Chem. 1978; 2415: 828-833.
Fossati, P., Prencipe, L. Serum triacylglycerols determined calorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982; 1: 2077-2080.
Allain, CC., Poon, LS, Chan, CS. Enzymatic determination of total serum cholesterol. ClinChem. 1974; 4: 470-475.
Burnstein, M., Selvenick, HR., Morfin, R. Rapid method for isolation of lipoprotein from human serum with polyanions. J Lipid Res., 1970; 11: 583- 395.
Friedewald, WT. Estimation of concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499-502.
Miranda KM,Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide.2001; 5: 62-71.
Beyaert R, Fiers W (1998): Tumor Necrosis Factor and Lymphotoxin. In Cytokines, A.R.M.-S. a. R. Thorpe, eds. Academic Press, San Diego, 1998; 335-360.
Nichans, WH., Samulelson, B. 1968. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem, 6: 126-30.
Lowry OH, Rosebrough NJ, Farr AL, Randall R: Proteinmeasurement with Folin’s phenol reagent. J BiolChem 1952;193: 265-275.
Jain WC: Schalm's Veterinary Hematology, ed 4, Lea and Febiger, Philadelphia. 1986, pp. 69 - 71.
Bancroft GD, Steven A. In. Theory and practice of histological technique 4th Ed. London; Churchill Livingstone. 1983; Pp. 99 – 112.
Abo-Allam, R.M., 2003. Data Statistical Analysis using SPSS Program. 1 Ed., Publication for 1stUniversities, Cairo.
Salgia, R., Hensing, T., Campbell, N. Personalized treatment of lung cancer. SeminOncol.2011; 38: 274–283.
Teixeira,V., Valente, H., Casal, S. Blood antioxidant and oxidative stress biomarkers acute responses to a 1000-m kayak sprint in elite male kayakers. J Sports Med physical fitness 2013; 53(1):71–79.
Boisseau, p., Loubaton, B. Nanomedicine, nanotechnology in medicine. C. R.Physique J. 2011; 620-630
Arrigoni, O., De Tullio, M.C. Ascorbic acid: much more than just an antioxidant. BiochimBiophysActa, 2002; 1569: 1-9.
Chan-Yeung, M., Ferreira, P., Frohlich, J., Schulzer, M., Tan, F. The effects of age, smoking, and alcohol on routine laboratory tests. Am. J. Clin. Pathol. 1981; 75(3): 320-326.
Cheung, B. M., Ong, K. L.,Wong, L. Y. Elevated serum alkaline phosphatase and peripheral arterial disease in the United States National Health and Nutrition Examination Survey 1999–2004. Int. J. Cardiol. 2009; 135(2): 156-161.
Wannamethee, S. G., Lowe, G. D., Shaper, A. G., Rumley, A., Lennon, L., Whincup, P. Associations between cigarette smoking, pipe/cigar smoking, and smoking cessation, and haemostatic and inflammatory markers for cardiovascular disease. Eur. Heart J. 2005; 26(17):1765-1773.
Friedman, L. S., Martin, P., Munoz, S. J. (1996). Liver function tests and the objective evaluation of the patient with liver disease, In: Zakin D, boyer TD, eds. Hepatology: A text book of liver disease (Vol. 41). Philadelphia: PA: WB saunders.
M. Iqbal, Y. Okazaki, and S. Okada, “In vitro curcumin modulates ferric nitrilotriacetate (Fe-NTA) and hydrogen peroxide (H2O2)-induced peroxidation of microsomal membrane lipids and DNA damage,”Teratogenesis Carcinogenesis and Mutagenesis, 2003; 23, 151–160.
L. M. G. Antunes, M. C. P. Araújo, J. D. C. Darin, and M. D. L. P. Bianchi, “Effects of the antioxidants curcumin and vitamin C on cisplatin-induced clastogenesis in Wistar rat bone marrow cells,” Mutation Research, 2000; 465: 131–137.
K. I. Priyadarsini, D. K. Maity, G. H. Naik et al., “Role of phenolic O-H and methylene hydrogen on the free radical reactions and antioxidant activity of curcumin,” Free Radical Biology and Medicine, 2003; 35: 475–484.
S. Awasthi, U. Pandya, S. S. Singhal et al., “Curcumin-glutathione interactions and the role of human glutathione S- transferase P1-1,” Chemico-Biological Interactions, 2000; 128: 19–38.
Pari L., Prasath A. Efficacy of caffeic acid inpreventing nickel induced oxidative damage in liverof rats. Chem. Biol. Interact. 2008; 173, 77-83.
Santra A, Chowdhury A, Ghatak S, Biswas A, Dhali GK. Arsenic induces apoptosis in mouse liver is mitochondria dependent and is abrogated by Nacetylcysteine. Toxicol. Appl. Pharmacol, 2007; 220: 146–155.
Chattopadhyay K, Mandal S, Chattopadhyay BD, Ghosh S. Ameliorative effect of sesame lignans on nicotine toxicity in rats. Food Chem Toxicol 2010; 48: 3215–3220.
Sinha S, Maiti M, Chattopadhyay K, Chattopadhyay BD. Potential amelioration of curcumin against nicotine-induced toxicity of protein malnourished female rats. J Pharmacol Toxicol. 2012; 4: 166-180.
Masora E J. Lipids and lipid metabolism, Annu Rev Physiol, 1977; 39: 301-308.
Brunzell, J. D, Miller N E, Alaupovic P, St. Hilaire R J., Wang C S. Familial chylomicronemia due to a circulation inhibitor of lipoprotein lipase activity, J Lipid Res, 1983; 24: 12-17.
Kim, M, KimY. Hypocholesterolemic effects of curcumin via up-regulation of cholesterol 7a-hydroxylase in rats fed a high fat diet. Nutr. Res. Pract., 2010; 4: 191-195.
Hasimun, P., Sukandar, EY, Adnyana IK, Tjahjono, DH. Synergistic effect of curcuminoid and s-methyl cysteine in regulation of cholesterol homeostasis. Int. J. Pharmacol., 2011; 7: 268-272.
Arafa, H.M. Curcumin attenuates diet induced hypercholesterolemia in rats. Med. Sci. Monit., 2005; 11: 228-234.
Asai, A, Miyazawa T. Dietary curcuminodoids prevent high-fat diet-induced lipid accumulation in rat liver and epididymal adipose tissue. J. Nutr., 2001; 131: 2932-2935.
Chung C. L., Ting T. O., Hui P. H, Chau J. W.The inhibition of oleic acid induced hepatic lipogenesis and the promotion of lipolysis by caffeic acid via up-regulation of AMP-activated kinase .2013;55:122-199.
Codrington, A. M. ; Hales, B. F, Robaire, B.: anti lipid oxidation of caffeic acid. 2007; 10-1095.
Lin CC, Yin MC, Hsu CC, Lin MP. Effect of five cysteine-containing compounds on three lipogenic enzymes in Balb/cA mice consuming a high saturated fat diet. Lipids. 2004;39:843–848.
Krieger MH, Santos KF, Shishido SM, Wanschel AC, Estrela HF, Santos L, De Oliveira MG, Franchini KG, Spadari-Bratfisch RC, Laurindo FR. Antiatherogenic effects of S-nitroso-N-acetylcysteine in hypercholesterolemic LDL receptor knockout mice. Nitric Oxide. 2006;14:12–20.
Diniz YS, Rocha KK, Souza GA, Galhardi CM, Ebaid GM, Rodrigues HG, Novelli Filho JL, Cicogna AC, Novelli EL. Effects of N-acetylcysteine on sucrose-rich diet-induced hyperglycaemia, dyslipidemia and oxidative stress in rats. Eur J Pharmacol. 2006;543:151–157.
Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287.
Brizzi P, Tonolo G, Carusillo F, Malaguarnera M, Maioli M, Musumeci S. Plasma lipid composition and LDL oxidation. Clin Chem Lab Med. 2003;41:56–60
Lin CC, Yin MC. Effects of cysteine-containing compounds on biosynthesis of triacylglycerol and cholesterol and anti-oxidative protection in liver from mice consuming a high-fat diet. Br J Nutr.2008;99:37–43.
Mahapatra, S.K., Das, S, Bhattacharjee, S, Gautam, N, Majumdar S, Roy, S. 2009. In vitro nicotine-induced oxidative stress in mice peritonial macrophages: A dose-dependant approach. Toxicol. Mech. Methods, 2009’ 19: 100-108.
Yildiz, D., Ercal N, Armstrong, DW. Nicotine enantiomers and oxidative stress. Toxicology, 1998; 130: 155-165.
Chattopadhyay, K, Chattopadhyay, B. Effect of nicotine on lipid profile, peroxidation and antioxidant enzymes in female rats with restricted dietary protein. Indian J. Med. Res., 2008; 127: 571-576.
Grester, H. β-Carotine, vitamin E and vitamin C in different stages of carcinogenesis. Eur. J. Clin. Nutr., 1995; 149: 155-168.
Sener, G., Toklu, HZ, Cetinel, S. β-Glucan protects against chronic nicotine-induced oxidative damage in rat kidney and bladder. Environ. Toxicol. Pharmacol., 2007; 23: 25-32.
Sreekala, S, Indira M. Effects of exogenous selenium on nicotine-induced oxidative stress in rats. Biol. Trace Elem. Res., 2009; 130: 62-71.
Zava, D.T., Dollbaum CM, Blen M. Estrogen and progestin bioactivity of foods, herbs and spices. Proc. Soc. Exp. Biol. Med., 1998; 217: 369-378.
Chimi H., Cillard J., Cillard P., Rahmani M. Peroxyl and hydroxyl radical scavenging activity ofsome natural phenolic antioxidants. J. Am. Oil.Chem. Soc. 1991; 68, 307-312.
Gurer H, Ozunes H, Neal R, Spitz DR, Ercal N. Antioxidant effects of N-acetylcysteine and succimer in red blood cells from lead exposed rats, Toxicol.1998;120:181-9.
Hemalatha P, Reddy AG, Reddy YR, Shivakumar P. Evaluation of protective effect of N-acetyl cysteine on arsenic-induced hepatotoxicity. J Nat Sc Biol Med, 2013; 4:393-5.
Sreejayan, N, Rao,MN. Free radical scavenging activity of curcuminoids.Arzneimittelforschhung. 1996; 46, 169–171.
Ghoneim, A.I. Effects of curcumin on ethanol-inducedhepatocyte necrosis and apoptosis: implication of lipid peroxidation and cytochrome c. Naunyn Schmiedebergs Arch Pharmacol .2009; 379:47–60.
Wang, W.Z., Cheng, J., Luo, J., Zhuang, S.M. Abrogation of G2/M arrest sensitizes curcumin-resistant hepatoma cells to apoptosis. FEBS Lett. 2008; 582:2689–95.
Lee Y., Shin D., Kim K.H., Hong S., Choi D., Kim Y.J., Kwak M.K., Jung Y. Caffiec acidphenethyl ester-mediated Nrf2 activation and IκBkinase inhibition are involved in NFκB inhibitoryeffect: Structural analysis for NFκB inhibition.European Journal of Pharmacology, 2010; 643,21 -28.
Jain, S.K., Kannan, K., Lim, G., McVie, R., Bocchini Jr., J.A. Hyperketonemia increases tumor necrosis factor-alpha secretion in cultured U937 monocytes and Type 1 diabetic patients and is apparently mediated by oxidative stress and cAMP deficiency. Diabetes 2002; 51, 2287–2293.
Ohta, M.Y., Nagai, Y., Takamura, T., Nohara, E., Kobayashi, K. Inhibitory effect of troglitazone on TNF-alpha-induced expression of monocyte chemoattractant protein-1 (MCP-1) in human endothelial cells. Diabetes Res. Clin. Pract. 2000; 48, 171–176.