Nutritional quality assessment and antiplasmodial activity of Cajanus cajan (L.) Huth., Crescentia cujete L. and Myrianthus preussii Engl. from Akure, Southwestern Nigeria
Keywords:
Nutrition, Malaria, Cajanus cajan, Crescentia cujete, Myrianthus preussii, NigeriaAbstract
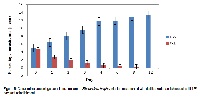
Background and Purpose of the Study: Many plants are now considered to have dual purpose usefulness in terms of their therapeutic effects and nutritional benefits. Cajanus cajan (L.) Huth., Crescentia cujete L. and Myrianthus preussii Engl. are combined for use in the treatment of malaria in Akure, Southwestern Nigeria. Materials and Methods: The powdered plant samples were screened for phytochemical constituents, proximate composition and mineral elements according to standard protocols. Plasmodium berghei infected mice were administered with water and ethanol extracts of plant samples and blood samples screened for parasitemia. Data were statistically analyzed. Results: Alkaloids, glycosides, saponins, tannins, and polyphenols were present in all the three samples. Anthraquinones and flavonoids were altogether absent. C. cajan had the highest ash (11.69%), crude protein (17.76%) and fat (17.34%) whereas C. cujete was richest in carbohydrate (58.52%). Calcium was found to be highest in C. cujete (22672.43mg/kg) and least in C. cajan (13288.33mg/kg). C. cujete was richest (898.37mg/kg) and C. cajan (304.22mg/kg) least in iron. However, magnesium was found to be highest in M. preussii (5837.03mg/kg) and least in C. cujete (2166.48mg/kg). The ethanol extract of the recipe was most active at 200mg/kg. Conclusions and Application of Findings: Dietary or mineral elements serve structural, functional and biochemical roles. The three plants contained appreciable major and minor elements. The leaf of C. cajan could serve as a complement for animal protein. The activity observed in the ethanol extract could be as a result of the complete dissolution of the phytochemicals in ethanol. Toxicity studies on the plants will confirm their safe application, although lead tested negative in the plant samples.
References
Abolaji OA, Adebayo AH, Odesanmi OS. Nutritional qualities of three medicinal plants (Xylopia aethiopica, Blighia sapida and Parinari polyandra) commonly used by pregnant women in the Western part of Nigeria. Pakistan J. Nutr. 2007; 6(6): 665-668.
Khalil E, Esoh R, Rababah T, Almajwal AM, Alu’datt MH. Minerals, proximate composition and their correlations of medicinal plants from Jordan. J. Med. Plts. Res. 2012; 6(47): 5757-5762.
Arukwe U, Amadi BA, Duru MKC, Agomuo EN, Adindu EA, Odika PC, Lele KC, Egejuru L, Anudike J. Chemical composition of Persea americana leaf, fruit and seed. IJRRAS. 2012; 11(2): 346-349.
Bouba AA, Njintang NY, Foyet HS, Scher J, Montet D, Mbofung CMF. Proximate composition, mineral and vitamin content of some wild plants used as spices in Cameroon. Food Nutr. Sci. 2012; 3: 423-432.
Nmorsi OPG, Ukwandu NCD, Egwunyenga AO. Antioxidant status of Nigerian children with Plasmodium falciparum malaria. Afr. J. Microb. Res. 2007; 61-64.
Nguta JM, Mbaria JM, Gakuya DW, Gathumbi PK, Kiama SG. Antimalarial herbal remedies of Msambweni, Kenya. J. Ethnopharmacol. 2010; 128: 424-432.
World Health Organization (WHO). Severe Falciparum malaria. Trans. Royal Soc. Trop. Med. Hyg. 94(Suppl. 1): 2000; 551-590.
Rowe AK. The burden of malaria among African children in the year 2000. Intl. J. Epidemiol. 2006; 35: 691-704.
Ayoola GA, Coker HAB, Adesegun SA, Adepoju-Bello AA, Obaweya K, Ezennia, Atangbayila TO. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in Southwestern Nigeria. Tropical J. Pharm. Res. 2008; 7(3): 1019-1024.
Ahsan MR, Islam KM, Haque ME, Mossaddik MA. In vitro antibacterial screening and toxicity study of some different medicinal plants. World J. Agric. Sci. 2009; 5(5): 617-621.
Patnaik A, Mohanty BK. Toxic effect of mercury and cadmium on germination and seedling growth of Cajanus cajan L. (Pigeon Pea). Annals Biol. Res. 2013; 4(3): 123-126.
Akande KE, Abubakar MM, Adegbola TA, Bogoro SE, Doma UD. Chemical evaluation of the nutritive quality of pigeon pea [Cajanus cajan (L.) Millsp.]. Intl. J. Poultry Sci. 2010; 9(1): 63-65.
Singh U, Jambunathan R, Saxena KB, Subrahmanyam N. Nutritional quality evaluation of newly developed high profile lines of pigeon pea (Cajanus cajan L.). J. Sci. Food Agric. 1990; 50: 201-209.
Ejelonu BC, Lasisi AA, Olaremu AG, Ejelonu OC. The chemical constituents of calabash (Crescentia cujete). Afr. J. Biotech. 2011; 10(84): 19631-19636.
Shastry CS, Bhalodia MM, Aswathanarayana BJ. Antivenom activity of ethanolic extract of Crescentia cujete fruit. Intl. J. Phytomed. 2012; 4: 108-114.
Burkill HM. The useful plants of Tropical West Africa. 2nd Edition, Richmond, UK, Kew Royal Botanical Garden, London. 1985.
Michael A. Trees, shrubs and lianas of West Africa dry zones. Grad Margae Publishers GMBH, MNHN. 2004; 191pp.
Agwa OK, Chuku W, Obichi EA. The in vitro effect of Myrianthus arboreus leaf extract on some pathogenic bacteria of clinical origin. J. Microbiol. Res. 2011; 1(4): 77-85.
Oyeyemi SD, Arowosegbe S, Adebiyi AO. Phytochemical and proximate evaluation of Myrianthus arboreus P. Beau. and Spargonophorus sporgonophora L. leaves. IOSR J. Agric. Vet. Sci. 2014; 7(9): 1-5.
National Bureau of Statistics (NBS). National Bureau of Statistics, National Population Commission, Abuja, Nigeria. 2006.
Folayan JA. Socio-economic analysis of Fadama farmers in Akure South Local Government Area of Ondo State, Nigeria. American J. Hum. Soc. Sci. 2013; 1(1): 10-17.
Walsh LM. Instrumental methods for analysis of soils and plant tissue. Soil Science Society of America Inc. Madison Wisconsin, USA. 1971.
Harbone JB. Phytochemical Methods. Chapman and Hall Ltd., London.1973; 49-188.
Association of Analytical Chemists (AOAC). Official Methods of Analysis. Association of Analytical Chemists. 15th Edition. Washington, DC, USA. 1990; 112-135.
Evans WC. Trease and Evans Pharmacognosy. 15th Edition. W.B. Saunders, London. 2002; 214-393.
Sofowora A. Medicinal plants and traditional medicine in Africa. 3rd Edition. Spectrum Books Ltd., Ibadan, Nigeria. 2008; 199-204.
Lorke D. A new approach to practical acute toxicity testing. Arch. Toxicol. 1983; 54: 275-287.
Hilou H, Nacoulma OG, Guiguemde TR. In vitro antimalarial activity of extracts of Amaranthus spinosus L. and Boerhavia erecta L. in mice. J. Ethnopharmacol. 2006; 103: 235-240.
Sonibare MA, Jayeola AA, Egunyomi A. Chemotaxonomic significance of leaf alkanes in species of Ficus (Moraceae). Biochem. Syst. Ecol. 2005; 33: 79-86.
Nwabuisi C. Prophylactic effect of multi-herbal extract “agbo-iba” on malaria induced in mice. East Afr. Med. J. 2002; 79(7): 343-346.
Benoit-Vicala F, Valentine A, Cournaca V, Pellisierb Y, Malliea M, Bastidea JM. In vitro antiplasmodial activity of stem and root extracts of Nauclea latifolia Sm. (Rubiaceae). J. Ethnopharmacol. 1998; 61: 173-178.
Kayode J. Conservation of indigenous medicinal botanicals in Ekiti State, Nigeria. J. Zheijang Univ. Sci. 2006; 23(5): 713-718.
Edeogu CO, Ezeonu FC, Okaka ANC, Ekuma CE, Elom SO. Proximate composition of staple food crops in Ebonyi State (South Eastern Nigeria). Intl. J. Biotech. Biochem. 2007; 3(1): 1-8.
Onwuka GI. Food analysis and instrumentation: theory and practice. Naphtalic Prints, Surulere, Lagos, Nigeria. 2005; 63-230.
Akpaibo UD, Ikpe EE. Proximate composition and nutrient analysis of Aneilema aequinoctiale leaves. Asian J. Plt Sci. Res. 2013; 3(2): 55-61.
Bhattacharjee S, Sultana A, Sazzad MH, Islam MA, Ahtasho, MM, Asaduzzaman. Analysis of the proximate composition and energy values of two varieties of Onion (Allium cepa L.) bulbs of different origin: A comparative study. Intl. J. Nutr. Food Sci. 2013; 2(5): 246-253.
Iorgyer MI, Odoh OE, Ikondo ND, Okoh JJ. The replacement value of pigeon pea (Cajanus cajan) for maize on performance of broiler finishers. Prod. Agric. Tech. 2009; 5(1): 67-74.
Amaefule KU, Obioha FC. Performance and nutrient utilization of broiler starters fed diets containing raw, boiled, and dehulled pigeon pea seeds. Nigerian J. Animal Prod. 2001; 28(1): 31-39.
Brody T. Nutritional Biochemistry. Academic Press, San Diego. 1994.
Mayer J, Goldberg J. Dr. Jean Mayer’s diet and nutrition guide. Pharos Books, New York. 1990.
Soetan KO, Olaiya CO, Oyewole OE. The importance of mineral elements for humans, domestic animals and plants: A review. Afr. J. Food Sci. 2010; 4(5): 200-222.
Anonymous. Elements comprising the human body: the roles of minerals and elements in life processes. www.mii.org/periodic/LifeElement.html. 2014; Accessed: 26th August, 2014.