Anti-inflammatory activity of phenolic extracts from different parts of prickly pear on lipopolysaccharide-stimulated N13 microglial cells
Keywords:
Prickly pears, phenolic extracts, microglia, LPS, anti-inflammatory activityAbstract
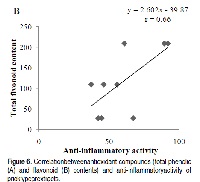
Phytochemicals with health-promoting activities that are components of human diet, have shown to exert a protective effect on the CNS under pathological conditions. In this sense, prickly pears exhibit analgesic and anti-inflammatory properties with neuroprotective effect. The purpose of this study was to evaluate the potential protective effect of phenolic extracts from different parts of prickly pear on the production of pro-inflammatory mediators by lipopolysaccharide (LPS) -stimulated N13 microglia. Activation of microglia, the hallmark of neuroinflammation, is key to host defence and tissue repair in brain. However, activated microglia secretes cytokines and other factors that are known to contribute to neurodegeneration. To preserve brain integrity, therefore, it is important to keep microglia activation under strict control. The results show that the extracts studied significantly inhibited the release of pro-inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α), interleukin 1-beta (IL-1β), and inducible nitric oxide synthase (iNOS). The present study, however, does not show a clear linear correlation between antioxidant compounds content (total phenolic and flavonoid contents) and anti-inflammatory activity indicates that there must be some additional components within the extracts that play a pivotal role in the anti-inflammatory effect and therefore further characterization is needed. The present study does, however, demonstrate that the phenolic extracts from different parts of prickly pears are potent inhibitors of microglial activation and thus a potential preventive therapeutic agent for neurodegenerative diseases involving neuroinflammation.
References
. Felker P, Rodriguez S, Casoliba RM, Filippini R, Medina D, Zapata R. Comparison of Opuntia ficus-indica varieties of Mexican and Argentine origin for fruit yield and quality in Argentina. J Arid Envir 2005; 60: 405-422.
. Habibi Y, Mahrouz M, Vignon MR. Microfibrillated cellulose from the peel of prickly pear fruits. Food Chem 2009; 115:423-429.
. Kuti JO. Antioxidant compounds from four Opuntia cactus pear fruit varieties. Food Chem 2004; 85: 527-533.
. Cayupán YSC, Ochoa MJ, Nazareno MA. Health promoting substances and antioxidant properties of Opuntia sp. fruits. Changes in bioactive-compound contents during ripening process. Food Chem 2011; 126: 514-519.
. Cejudo-Bastante MJ, Chaalal M, Louaileche H, Parrado P, Heredia FJ. Betalain profile, phenolic content, and colour characterization of different parts and varieties of Opuntia ficus-indica. J Agric Food Chem 2014; 62: 8491-8499.
. Magloire JF, Konarski P, Zou D, Stintzing FC, Zou CH. Nutritional and medicinal uses of cactus pear (Opuntia spp.) cladodes and fruits. Frontiers in Biosc 2006; 11: 2574-2589.
. Abd El-Raze FH, Hassan AA. Nutritional value and hypoglycemic effect of prickly cactus pear (Opuntia ficus-indica) fruit juice in Alloxan-induced diabetic rats. Aust J Basic Appl Sci. 2011; 10: 356-377.
. Yeddes N, Chérif JK, Guyot S, Sotin H, Ayadi MT. Comparative study of antioxidant power, polyphenols, flavonoids and betacyanins of the peel and pulp of three Tunisian Opuntia forms. Antioxidants 2013; 1: 37-51.
. Kaushik DK, Basu AA. Friend in need may not be a friend indeed: role of microglia in neurodegenerative diseases. CNS Neurol Disord Drug Targets 2013; 12: 726-740.
. Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010; 7: 354-365.
. Park EH, Kahng JH, Paek EA. Studies on the pharmacological actions of cactus: identification of its anti-inflammatory effect. Arch Pharm Res 1998; 21: 30-34.
. Park EH, Kahng JH, Lee SH, Shin KH. An anti-inflammatory principle from cactus. Fitoterapia 2001; 72: 288-290.
. Matias A, Nunes SL, Poejo J, Mecha E, Serra AT, Madeira PJA, Bronze MR, Duarte CMM. Antioxidant and anti-inflammatory activity of a flavonoid-rich concentrate recovered from Opuntia ficus-indica juice. Food Funct 2014; 5 (12): 3269-3280.
. Hyang DG, Kwang HL, Hyoung JK, Eun HL, Jiyong L, Yun SS, Yong-Ha L, Changbae J, Yong SL, Jungsook C. Neuroprotective effects of antioxidative flavonoids, quercetin, (1)-dihydroquercetin and quercetin 3-methyl ether, isolated from Opuntia ficus-indica var. saboten. Brain Res 2003; 965: 130-136.
. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enolo Vitic 1965; 16:144-158.
. Quettier-Deleu C, Gressier B, Vasseur J, Dine T, Brunet C, Luyckx M, Cazin M, Cazin JC, Bailleul F, Trotin F. Phenolic compounds and antioxidant activities of buckweat (Fagopyrum esculentum Moench) hulls and flour. J Ethnopharm 2000; 72: 35-42.
. Gavilán MP, Castaño A, Torres M, Revilla E, Caballero C, Jiménez S, García-Martínez A, Parrado J, Vitorica J, Ruano D. Age-related increase in the immunoproteasome content in rat hippocampus: molecular and functional aspects. J Neurochem 2009; 108: 260-270.
. Palsson-McDermott EM, O’Neill LA. Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 2004; 113: 153-162.
. Hanisch UK. Microglia as a source and target of cytokines. Glia 2002; 40: 140-55.
. McGeer PL, McGeer EG. Inflammation and the degenerative diseases of aging. Ann NY Acad Sci 2004; 1035: 104-116.
. Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol 2008; 29: 357-365.
. Levites Y, Amit T, Youdim MB, Mandel S. Involvement of protein kinase C activation and cell survival/ cell cycle genes in green tea polyphenol (−)-epigallocatechin 3-gallate neuroprotective action. J Biol Chem 2002; 277: 30574-30580.
. Spencer JP. Flavonoids and brain health: multiple effects underpinned by common mechanisms. Genes Nutr 2009; 4: 243-250.
. Wang JY, Wen LL, Huang YN, Chen YT, Ku MC. Dual effects of antioxidants in neurodegeneration: direct neuroprotection against oxidative stress and indirect protection via suppression of glia-mediated inflammation. Curr Pharm Des 2006; 12: 3521-3533.
. Zhang L, Ravipati AS, Koyyalamudi SR, Jeong SC, Reddy N, Smith PT, Bartlett J, Shanmugam K, Munch G, Wu MJ. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J Agric Food Chem 2011; 59: 12361-12367.
. Chaalal M, Touati N, Louaileche H. Extraction of phenolic compounds and in vitro antioxidant capacity of prickly pear seeds. Acta Bot Gallica 2012; 159: 467-475.
. Chaalal M, Louaileche H, Touati N, Bey MB. Phytochemicals, in vitro antioxidant capacity and antiradical potential of whole and ground seeds of three prickly pear varieties: A comparative study. Ind Crops Prod 2013; 49: 386-391.
. Ruano D, Revilla E, Gavilan MP, Vizuete ML, Pintado C, Vitorica J, Castano A. Role of p38 and inducible nitric oxide synthase in the in vivo dopaminergic cells’ degeneration induced by inflammatory processes after lipopolysaccharide injection. Neuroscience 2006 ; 140 : 1157−1168.
. Pintado C, Revilla E, Vizuete ML, Jiménez S, García- Cuervo L, Vitorica J, Ruano D, Castaño A. Regional difference in inflammatory response to LPS-injection in the brain: role of microglia cell density. J Neuroimmunol 2011; 238 : 44-51.
. Candiracci M, Piatti E, Domínguez-Barragán M, García-Antrás D, Morgado B, Ruano D, Gutiérrez JF, Parrado J, Castaño A. Anti-inflammatory activity of a honey flavonoid extract on lipopolysaccharide-activated N13 microglial cells. J Agric Food Chem 2012; 60: 12304-12311.
. Saha RN, Pahan K. Regulation of inducible nitric oxide synthase gene in glial cells. Antioxid Redox Signal 2006; 8: 929-947.
. Lee MH, Kim JY, Yoon JH, Lim HJ, Kim TH, Jin C, Kwak WJ, Han CK, Ryu JH. Inhibition of nitric oxide synthase expression in activated microglia and peroxynitrite scavenging activity by Opuntia ficus indica var. saboten. Phytother Res. 2006; 20: 742-7.
. MacEwan, D.J., 2002. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal 2002; 14: 477-492.
. El-Mostafa K, El-Kharrassi Y, Badreddine A, Andreoletti P, Vamecq J, El Kebbaj MS, Latruffe N, Lizard G, Nasser B, Cherkaoui-Malki M. Nopal Cactus (Opuntia ficus-indica) as a source of bioactive compounds for nutrition, health and disease. Molecules 2014; 19: 14879-14901.
. Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol 2006 ; 72 : 1439-1452.